Question: In Problem 12.54, you calculated the CO 2 emission factor for several hydrocarbon fuels. Derive an expression for the CO 2 emission factor for an
In Problem 12.54, you calculated the CO2 emission factor for several hydrocarbon fuels. Derive an expression for the CO2 emission factor for an arbitrary hydrocarbon CxHy in which the hydrogen-to-carbon ratio y/x appears explicitly. Assume that all the fuel carbon appears in the products as CO2 and use “simple” air. The atomic weights of C and H are 12.011 and 1.008, respectively. Use your expression to evaluate four of the same fuels as before:
i. Methane (CH4)
ii. Propane (C3H8)
iii. Gasoline (C8H15-equivalent)iv. Diesel fuel (C15H22-equivalent)Plot the CO2 emission factor as a function of the hydrogen-to-carbon ratio y/x.
Problem 12.54
Determine the mass of CO2 produced (kg) per unit of energy released (kJ), i.e., the CO2 emission, for the complete combustion of the following fuels. The higher heating value (HHV) expresses the energy released per mass of fuel burned.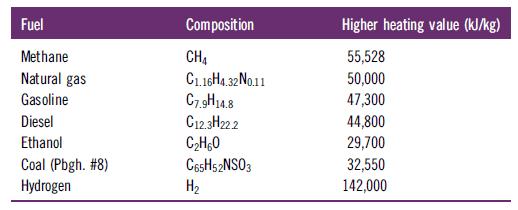
Fuel Methane Natural gas Gasoline Diesel Ethanol Coal (Pbgh. #8) Hydrogen Composition CH4 C1.16H4.32 No.11 C7.9H14.8 C12.3H222 CH60 C65H52NSO3 H Higher heating value (kJ/kg) 55,528 50,000 47,300 44,800 29,700 32,550 142,000
Step by Step Solution
3.55 Rating (162 Votes )
There are 3 Steps involved in it
To derive an expression for the CO2 emission factor for an arbitrary hydrocarbon CxHy we can start with the balanced chemical equation for the complet... View full answer

Get step-by-step solutions from verified subject matter experts


