A chemical plant produces oxygen by liquifying air and separating it into its component gases by fractional
Question:
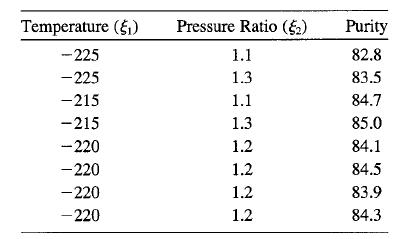
A chemical plant produces oxygen by liquifying air and separating it into its component gases by fractional distillation. The purity of the oxygen is a function of the main con-denser temperature and the pressure ratio between the upper and lower columns. Current operating conditions are temperature (ξl) = -220°C and pressure ratio (ξ2) = 1.2. Using the following data, find the path of steepest ascent:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





