One mole of a hydrocarbon fuel (CH x ) is burned with excess air. The volumetric analysis
Question:
One mole of a hydrocarbon fuel (CHx) is burned with excess air. The volumetric analysis of the dry products (with H2O removed) yields: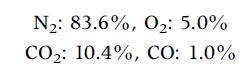
A. Determine the approximate composition of the fuel on a mass basis.B. Determine the percent theoretical air.C. Determine the equivalence ratio.
Transcribed Image Text:
N₂: 83.6%, O₂: 5.0% CO₂: 10.4%, CO: 1.0%
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
A To determine the approximate composition of the fuel on a mass basis we need to do a mass balance on the carbon and hydrogen in the fuel Since we do...View the full answer

Answered By

Diana Muriuki
As an online math tutor, I have several years of hands-on experience working with students of all ages and skill levels. I hold a Bachelor's degree in Mathematics and a Master's degree in Education. Additionally, I have completed multiple training courses in online teaching and tutoring methods.
Throughout my career, I have worked with students in both individual and group settings, including classroom teaching, after-school tutoring, and online instruction. I am proficient in teaching a wide range of math topics, from basic arithmetic to advanced calculus and statistics.
One of my greatest strengths as a tutor is my ability to adapt my teaching style to meet the unique needs and learning styles of each individual student. I understand that every student is different, and I strive to create a comfortable and supportive learning environment that encourages growth and development.
In addition to my formal education and tutoring experience, I am also a lifelong learner with a passion for mathematics. I am constantly seeking out new resources and methods to improve my own knowledge and skills, and I believe this passion and enthusiasm helps to inspire my students as well.
Overall, my hands-on experience and proficiency as a math tutor are grounded in a combination of formal education, practical experience, and a genuine love of mathematics. I am confident in my ability to help students achieve their goals and succeed in math, and I look forward to the opportunity to work with new students and continue to grow as an educator.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
The combustion of a hydrocarbon fuel with air results in a mixture of products of combustion having the composition on a volume basis as follows: 4.89 percent carbon dioxide, 6.50 percent water...
-
A hydrocarbon fuel CaHb is burned with dry air. Determine the composition of the fuel combusted (whole number atoms) and the percent theoretical air if a dry volumetric analysis of the products...
-
n-Pentane is burned with excess air in a continuous combustion chamber. (a) A technician runs an analysis and reports that the product gas contains 0.270 mole% pentane, 5.3% oxygen, 9.1% carbon...
-
Consider a set of documents. Assume that all documents have been normalized to have unit length of 1. What is the "shape" of a cluster that consists of all documents whose cosine similarity to a...
-
Most airlines in the United States have frequent flier programs that grant free flights if a customer accumulates enough flight miles on the airline. For example, United Airlines offers a free...
-
Divide the following expression. 6 ???? 3 + 8 ???? 2 - 1 0 ???? 2 ????
-
A six cylinder four stroke petrol engine develops \(300 \mathrm{~kW}\) (BP) at \(2500 \mathrm{rpm}\). The stroke to bore ratio is 1.25. The mean effective pressure on each piston is 9 bar and...
-
On July 31, 2019, Keeds Company had a cash balance per books of $6,140. The statement from Dakota State Bank on that date showed a balance of $7,690.80. A comparison of the bank statement with the...
-
Miller Company's contribution format income statement for the most recent month is shown below: Total Sales (25,200 units) Variable expenses $ 226,800 136,080 Contribution margin 90,720 Per Unit $...
-
Extrapolation: The file swim.dat contains data on the amount of time, in seconds, it takes each of four high school swimmers to swim 50 yards. Each swimmer has six times, taken on a biweekly basis....
-
Ethane burns with 150% stoichiometric air. Assume the air is 79% N 2 and 21%O 2 by volume. Combustion goes to completion. Determine (a) The airfuel ratio by mass (b) The mole fraction (percentage) of...
-
Natural gas is burned to produce hot water to heat a clothing store. Assuming that the natural gas can be approximated as methane (CH 4 ) and that the air is a simple mixture of O 2 and N 2 in molar...
-
The information is based on the spreadsheet as shown Suppose Ingredient I is made up of \(80 \%\) micoden and \(20 \%\) water, Ingredient II is made up of \(30 \%\) micoden, \(50 \%\) bixon, and \(20...
-
What is the role of social networking in your job search?
-
What types of information should you include in your rsum?
-
Go to the following website that ranks the 10 most corrupt world leaders: http://www.infoplease.com/ipa/A0921295.html. Do you detect any empirical patterns from this list's data? Find and identify...
-
Write an email to your instructor summarizing your teams progress. In the introductory paragraph, summarize the teams progress in terms of its goals and its schedule, your own progress on the tasks...
-
Research a hot business communication topic from the news (e.g., health care benefits, ethics, the economy, job layoffs, communication technology). Find at least three sources for your topic. Then,...
-
Friggle Corp. is a leasing and property management company located in Alberta. It provides financing to organizations wishing to purchase equipment or property and manages apartments and condominium...
-
Nitrogen monoxide reacts with hydrogen as follows: 2NO(g)+ H2(g) N2O(g) + H2O(g) The rate law is [H2]/ t = k[NO]2[H2], where k is 1.10 107 L2/(mol2s) at 826oC. A vessel contains NO and H2 at...
-
Invoking the usual normality assumptions, find an expression for the probability that a negative estimate of a variance component will be obtained by the analysis of variance method. Using this...
-
Analyze the data in Problem 12-9, assuming that operators are fixed, using both the unrestricted and the restricted form of the mixed models. Compare the results obtained from the two models. Problem...
-
Consider the variance components in the random model from Problem 12-9. (a) Find an exact 95 percent confidence interval on 2 . (b) Find approximate 95 percent confidence intervals on the other...
-
The Rolling Department of Kraus Steel Company had 5,300 tons in beginning work in process inventory (20% complete) on October 1. During October, 88,800 tons were completed. The ending work in process...
-
The Chamberlain Corporation reported the following: Income Statement Year 3 Year 2 Year 1 Revenue 12,300 10,800 8,900 Cost of goods sold 10,200 9,600 6,400 Selling & admin, expenses 950 800 1.200 Net...
-
On January 2, 2021, Fuji Company bought a machine for use in operations. The machine has an estimated useful life of eight years and an estimated residual value of $1,550. The company provided the...

Study smarter with the SolutionInn App


