Repeat Problem 7.1 but for the case where 54 kJ of energy is removed from the system
Question:
Repeat Problem 7.1 but for the case where 54 kJ of energy is removed from the system in a heat interaction. Also discuss how your result would change if the process were irreversible rather than reversible.
Problem 7.1
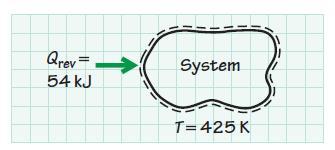
In a reversible heat interaction, 54 kJ of energy is transferred from the surroundings to a thermodynamic system. The process occurs isothermally at 425 K. Determine the entropy change of the system. How would the entropy of the system change if the process were irreversible rather than reversible?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:





