Determine the energy transfer as heat (J) required to change the state of 2 kg of methane
Question:
Determine the energy transfer as heat (J) required to change the state of 2 kg of methane from a saturated liquid at 1 atm to a gas at 300 K and 1 atm, assuming that the methane is confined at constant pressure during this process. Check your read of the tables using the P?h chart, and make a P?h process sketch showing the initial and final states, the vapor dome, and the line P = 1 atm. Use the tables in Appendix A.5 for data.
Data From Appendix A.5
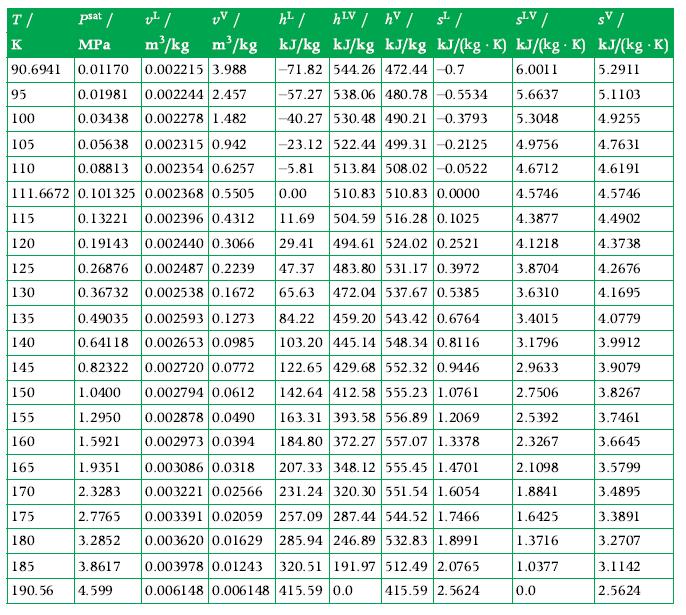
Transcribed Image Text:
T/ psat / K MPa 90.6941 0.01170 0.01981 0.03438 95 100 105 110 111.6672 0.101325 115 120 125 130 135 140 145 150 155 160 165 170 175 180 185 190.56 v²/ UV / m³/kg m³/kg 0.002215 3.988 0.05638 0.002315 0.942 0.08813 0.002354 0.6257 0.002368 0.5505 0.13221 0.002396 0.4312 0.19143 0.002440 0.3066 0.26876 0.002487 0.2239 0.36732 0.002538 0.1672 0.49035 0.002593 0.1273 0.64118 0.002653 0.0985 0.82322 0.002720 0.0772 1.0400 0.002794 0.0612 1.2950 0.002878 0.0490 1.5921 0.002973 0.0394 0.002244 2.457 0.002278 1.482 3.8617 4.599 h² / hLV / hv / s¹/ SLV / SV/ kJ/kg kJ/kg kJ/kg kJ/(kg .K) kJ/(kg. K) kJ/(kg .K) -71.82 544.26 472.44 -0.7 6.0011 5.2911 -57.27 538.06 480.78 -0.5534 -40.27 530.48 490.21 -0.3793 1.9351 207.33 348.12 555.45 1.4701 0.003086 0.0318 0.003221 0.02566 2.3283 231.24 320.30 551.54 1.6054 2.7765 0.003391 0.02059 257.09 287.44 544.52 1.7466 3.2852 0.003620 0.01629 285.94 246.89 532.83 1.8991 -23.12 522.44 499.31 -0.2125 4.9756 -5.81 513.84 508.02 -0.0522 4.6712 0.00 510.83 510.83 0.0000 4.5746 11.69 504.59 516.28 0.1025 4.3877 29.41 494.61 524.02 0.2521 4.1218 47.37 483.80 531.17 0.3972 3.8704 65.63 472.04 537.67 0.5385 3.6310 84.22 459.20 543.42 0.6764 3.4015 3.1796 103.20 445.14 548.34 0.8116 122.65 429.68 552.32 0.9446 2.9633 142.64 412.58 555.23 1.0761 2.7506 2.5392 163.31 393.58 556.89 1.2069 184.80 372.27 557.07 1.3378 2.3267 5.6637 5.3048 320.51 191.97 512.49 2.0765 0.003978 0.01243 0.006148 0.006148 415.59 0.0 415.59 2.5624 2.1098 1.8841 1.6425 1.3716 1.0377 0.0 5.1103 4.9255 4.7631 4.6191 4.5746 4.4902 4.3738 4.2676 4.1695 4.0779 3.9912 3.9079 3.8267 3.7461 3.6645 3.5799 3.4895 3.3891 3.2707 3.1142 2.5624
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
To determine the energy transfer as heat required to change the state of 2 kg of methane from a satu...View the full answer

Answered By

Monette Taban
I am currently studying Computer Science Engineering, Due to my interest in programming languages and coding, I am interesetd on Technology so I search about it read about different types of technologies, I think my this habbis will help me to solve problems of students and that is why I am signing as a question answer expert.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:
Students also viewed these Engineering questions
-
An alkali metal, potassium, is the working fluid in a nuclear power system that was proposed in the 1960s for space application, either on board of a spacecraft or in power stations on the Moon or...
-
Write a program Using C# Saudi Airlines permit each passenger to hold one bag. The maximum weight allowed for the bag depends on the class of the passenger which is: o 30 Kg for First class 'F'. o 25...
-
Liquid methane is to be converted into gas for insertion into the natural gas pipeline by a steady-state gasification plant. The methane will enter the plant as a saturated liquid at 1 atmosphere...
-
For the Kelvin state as considered in Example 15.4, explicitly justify the displacement and stress results given in relations (15.2.8) and (15.2.10). Data from example 15.4 Equation 15.2.8 Equation...
-
Project A requires a $280,000 initial investment for new machinery with a five-year life and a salvage value of $30,000. The company uses straight-line depreciation. Project A is expected to yield...
-
It is desirable to produce a copper-nickel alloy that has a minimum noncold-worked tensile strength of 350MPa (50,750psi) and a ductility of at least 48%EL. Is such an alloy possible? If so, what...
-
What are rites of passage and rites of intensification?
-
(Single-step Income Statement) The financial records of LeRoi Jones Inc. were destroyed by fire at the end of 2004. Fortunately the controller had kept certain statistical data related to the income...
-
ed Campbell Company established a predetermined variable overhead cost rate at $24.90 per direct labor hour. The actual variable overhead cost rate was $23.10 per hour. The planned level of labor...
-
On January 1, Year 4, Handy Company (Handy) purchased 70% of the outstanding common shares of Dandy Limited (Dandy) for $13,300. On that date, Dandy's shareholders' equity consisted of common shares...
-
A 2 m 3? container for heating water is initially filled with H 2 O liquid and vapor at 400 K, with 20% of the volume being liquid. Use the aatables in Appendix A.3 to work this exercise. Check your...
-
In order to illustrate the nature of the critical point, one can place CO 2 in a quartz vial and seal the device. Assuming that v c = 0.002 m 3 /kg, and that the device is filled at room temperature...
-
The Lewiston School District receives a $200,000 grant from the Bates Foundation to upgrade its high school computer labs. Total cost of the upgrade is estimated at $450,000. Because the grant is...
-
a. Explain how it is possible that the mine workers are being paid less than the wage that would be paid in a competitive labor market. b. Explain how the Escondida miners union might be able to...
-
How can Equity try to change the demand for female and minority labor?
-
Humans abuse the oceans. It is predicted that by 2050, overfishing, dumping waste, and heat from greenhouse-gas emissions will have destroyed most of the coral reefs and resulted in the oceans...
-
San Francisco led the movement to ban plastic shopping bags and is now proposing to ensure people have access to safe high-quality tap water and ban bottled water. A supporter of the ban says The...
-
a. Thinking about competition in game consoles as a game, describe the firms strategies. b. What, based on the information provided, turned out to be the equilibrium of the game? Sony, Microsoft, and...
-
You place 1.85 grams of wood in a vessel with 9.45 grams of air and seal the vessel. Then you heat the vessel strongly so that the wood burns. In burning, the wood yields ash and gases. After the...
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
Use MATLAB to calculate a. 6 tan -1 (12.5) + 4 b. 5 tan [3 sin -1 (13/5)] c. 5 ln(7) d. 5 log(7) Check your answers with a calculator.
-
The Richter scale is a measure of the intensity of an earthquake. The energy E (in joules) released by the quake is related to the magnitude M on the Richter scale as follows. E = 10 4.4 10 1.5M How...
-
Use MATLAB to nd the roots of 13 x 3 + 182x 2 - 184x + 2503 = 0.
-
Which economic system did European powers adhere to during the early modern period?
-
A licensee recently was placed on court - ordered probation. Does the licensee have to report this to the Board?
-
How are the monadic, the dyadic (relational) and the systemic (network) perspectives on exchanges interconnected? Use Social Exchange Theory to support your arguments.

Study smarter with the SolutionInn App


