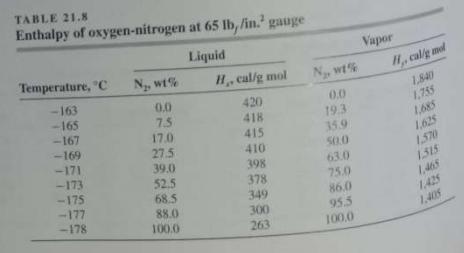
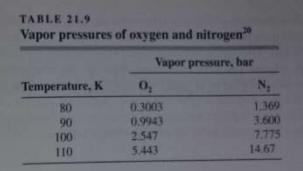
(a) Use the data in Tables 21.8 and 21.9 to calculate the relative volatility of the N...
Question:
(a) Use the data in Tables 21.8 and 21.9 to calculate the relative volatility of the N2-O2 system for different compositions at 65 lbƒ/in.2 gauge pressure.
(b) How close to an ideal system is this?
(c) Neglecting the effect of trace gases, what is the minimum number of ideal plates required to separate air into products of 99 percent purity?
Tables 21.8:
Tables 21.9:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Unit Operations Of Chemical Engineering
ISBN: 9780072848236
7th Edition
Authors: Warren McCabe, Julian Smith, Peter Harriott
Question Posted:





