The boiling point-equilibrium data for the system acetone-methanol at 760 mm Hg are given in Table 21.6.
Question:
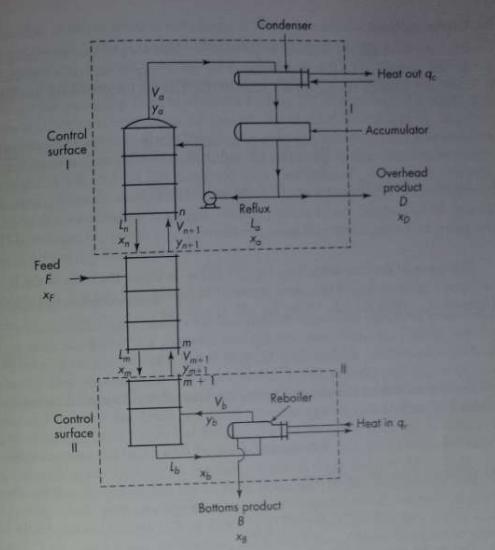
The boiling point-equilibrium data for the system acetone-methanol at 760 mm Hg are given in Table 21.6. A column is to be designed to separate a feed analyzing 25 mol % acetone and 75 mol % methanol into an overhead product containing 78 mol % acetone and a bottom product containing 1.0 mole percent acetone. The feed enters as an equilibrium mixture of 30 percent liquid and 70 percent vapor. A reflux ratio equal to twice the minimum is to be used. An external reboiler is to be used. Bottom product is removed from the reboiler. The condensate (reflux and overhead product) leaves the condenser at 25°C, and the reflux enters the column at this tem- perature. The molal latent heats of both components are 7,700 g cal/g mol. The Murphree plate efficiency is 70 percent. Calculate
(a) The number of plates required above and below the feed;
(b) The heat required at the reboiler, in Btu per pound mole of overhead product;
(c) The heat removed in the condenser, in Btu per pound mole of overhead product.
Table 21.6
Step by Step Answer:

Unit Operations Of Chemical Engineering
ISBN: 9780072848236
7th Edition
Authors: Warren McCabe, Julian Smith, Peter Harriott





