Compare the entropy change of the warmer water to that of the colder water during one cycle
Question:
(a) The entropy does not change during one cycle in either case.
(b) The entropy of both increases, but the entropy of the colder water increases by more because its initial temperature is lower.
(c) The entropy of the warmer water decreases by more than the entropy of the colder water increases, because some of the heat removed from the warmer water goes to the work done by the engine.
(d) The entropy of the warmer water decreases by the same amount that the entropy of the colder water increases.
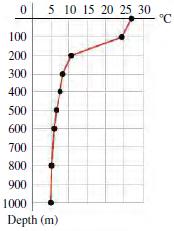
Ocean thermal energy conversion is a process that uses the temperature difference between the warm surface water of tropical oceans and the cold deep ocean water to run a heat engine. The graph shows a typical decrease of temperature with depth below the surface in tropical oceans. In the heat engine, the warmer surface water vaporizes a low-boiling-point fluid, such as ammonia. The heat of vaporization of ammonia is 260 cal/g at 27°C, the surface-water temperature. The vapor is used to turn a turbine and is then condensed back into a liquid by means of cold water brought from deep below the surface through a large intake pipe. A power plant producing 10 MW of useful power would require a cold seawater flow rate of about 30,000 kg/s.
Step by Step Answer:

University Physics with Modern Physics
ISBN: 978-0133977981
14th edition
Authors: Hugh D. Young, Roger A. Freedman





