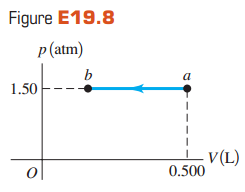
Figure E19.8 shows ap V-diagram for an ideal gas in which its absolute temperature at bis one-fourth
Question:
(a) What volume does this gas occupy at point b?
(b) How many joules of work was done by or on the gas in this process? Was it done by or on the gas?
(c) Did the internal energy of the gas increase or decrease from a to b? How do you know?
(d) Did heat enter or leave the gas from a to b? How do you know?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

University Physics with Modern Physics
ISBN: 978-0321696861
13th edition
Authors: Hugh D. Young, Roger A. Freedman, A. Lewis Ford
Question Posted:





