Question: Many biologically important molecules are optically active. When linearly polarized light traverses a solution of compounds containing these molecules, its plane of polarization is rotated.
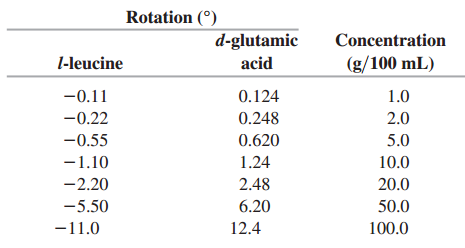
From these data, find the relationship between the concentration C (in grams per 100 mL) and the rotation of the polarization (in degrees) of each amino acid.
Rotation () d-glutamic Concentration l-leucine acid (g/100 mL) -0.11 0.124 1.0 -0.22 0.248 2.0 -0.55 0.620 5.0 1.24 -1.10 10.0 20.0 -2.20 2.48 6.20 -5.50 50.0 -11.0 12.4 100.0
Step by Step Solution
3.49 Rating (175 Votes )
There are 3 Steps involved in it
Identify The angle by which the plane of polarization of light is rotated depends on the concentrati... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1477_605aea4cb111b_682294.pdf
180 KBs PDF File
1477_605aea4cb111b_682294.docx
120 KBs Word File


