(See Exercise 21.21.) (a) Calculate the electric potential energy of the adeninethymine bond, using the same combinations...
Question:
(a) Calculate the electric potential energy of the adenine€“thymine bond, using the same combinations of molecules (O-H-N and N-H-N) as in Exercise 21.21.
(b) Compare this energy with the potential energy of the proton€“electron pair in the hydrogen atom.
Data from exercise 21.21
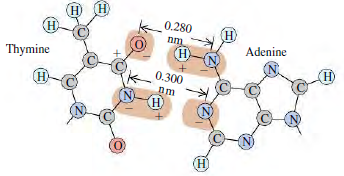
The two sides of the DNA double helix are connected by pairs of bases (adenine, thymine, cytosine, and guanine). Because of the geometric shape of these molecules, adenine bonds with thymine and cytosine bonds with guanine. Figure E21.21 shows the bonding of thymine and adenine. Each charge shown is ±e, and the H€”N distance is 0.110 nm.
Figure E21.21
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

University Physics with Modern Physics
ISBN: 978-0133977981
14th edition
Authors: Hugh D. Young, Roger A. Freedman
Question Posted:





