Question: Van der Waals bonds arise from the interaction between two permanent or induced electric dipole moments in a pair of atoms or molecules.(a) Consider two
Van der Waals bonds arise from the interaction between two permanent or induced electric dipole moments in a pair of atoms or molecules.(a) Consider two identical dipoles, each consisting of charges +q and ?? q separated by a distance d and oriented as shown in Fig. 42.40a. Calculate the electric potential energy, expressed in terms of the electric dipole moment p = qd, for the situation where r? d. Is the interaction attractive or repulsive, and how does this potential energy vary with T, the separation between the centers of the two dipoles? (b) Repeat part (a) for the orientation of the dipoles shown in Fig. 42.40b. The dipole interaction is more complicated when we have to average over the relative orientations of the two dipoles due to thermal motion or when the dipoles are induced rather thanpermanent.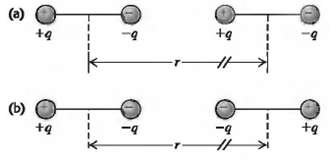
(b) +q +q -9 +q H -9 b- +q
Step by Step Solution
3.33 Rating (165 Votes )
There are 3 Steps involved in it
a U ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
P-M-P-M-C-M (52).docx
120 KBs Word File


