The free expansion of an ideal gas is an adiabatic process and so no heat is transferred.
Question:
Eq.(20.19)
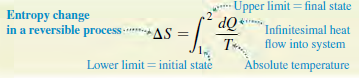
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

University Physics with Modern Physics
ISBN: 978-0133977981
14th edition
Authors: Hugh D. Young, Roger A. Freedman
Question Posted:





