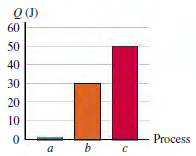
You have recorded measurements of the heat flow Q into 0.300 mol of a gas that starts
Question:
(a) Identify each process a, b, or c as isobaric, isochoric, or adiabatic.
(b) What is the value of T2?
(c) How much work is done by the gas in each process?
(d) For which process is the magnitude of the volume change the greatest?
(e) For each process, does the volume of the gas increase, decrease, or stay the same?
Figure P19.59
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

University Physics with Modern Physics
ISBN: 978-0133977981
14th edition
Authors: Hugh D. Young, Roger A. Freedman
Question Posted:





