Question: Suppose that the paths AD and BC represent adiabatic processes. What then are the work done by the gas and the heat absorbed by the
Suppose that the paths AD and BC represent adiabatic processes. What then are the work done by the gas and the heat absorbed by the gas in following the path ABC?
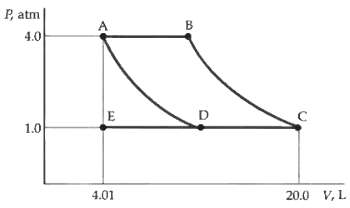
P, atm 4.0 1.0 4.01 20.0 V, L.
Step by Step Solution
3.48 Rating (174 Votes )
There are 3 Steps involved in it
a T is a state function See Problem 81 for T A T C T A 648 K T C 810 K b We find ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
10-P-T-F-L (558).docx
120 KBs Word File


