Question: The difference between the specific heats at constant pressure and volume is described by the expression where ?v is the volume coefficient of thermal expansion,
The difference between the specific heats at constant pressure and volume is described by the expression
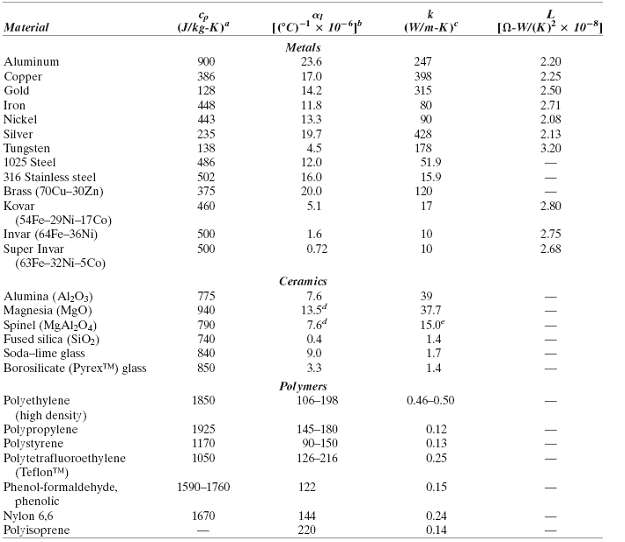
 where ?v is the volume coefficient of thermal expansion, v0 is the specific volume (i.e., volume per unit mass, or the reciprocal of density), ? is the compressibility, and T is the absolute temperature. Compute the values of cv at room temperature (293 K) for copper and nickel using the data in Table 19.1, assuming that ?v = 3?l and given that the values of ? for Cu and Ni are 8.35 x 10-12 and 5.51 x 10-12 (Pa)-1, respectively.
where ?v is the volume coefficient of thermal expansion, v0 is the specific volume (i.e., volume per unit mass, or the reciprocal of density), ? is the compressibility, and T is the absolute temperature. Compute the values of cv at room temperature (293 K) for copper and nickel using the data in Table 19.1, assuming that ?v = 3?l and given that the values of ? for Cu and Ni are 8.35 x 10-12 and 5.51 x 10-12 (Pa)-1, respectively.
(19.10)
Step by Step Solution
3.45 Rating (168 Votes )
There are 3 Steps involved in it
This problem asks that we calculate the values of c v for co... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (750).docx
120 KBs Word File


