Question: The distribution coefficient for extraction of a metal complex from aqueous to organic solvents is D = [total metal] org /[total metal] aq . Give
The distribution coefficient for extraction of a metal complex from aqueous to organic solvents is D = [total metal]org/[total metal]aq. Give physical reasons why β and Ka appear in the numerator of Equation 22-13, but KL and [H+]aq appear in the denominator.
Equation 22-13
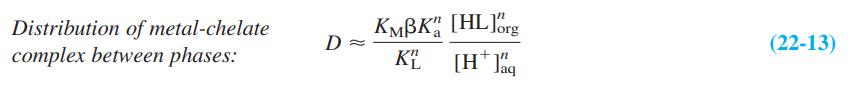
KMBK", [HL]brg Distribution of metal-chelate complex between phases: (22-13) KE [H* J%q
Step by Step Solution
3.58 Rating (169 Votes )
There are 3 Steps involved in it
The form that is extracted into organic solvent is MLW The formation of ML n is f... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2301).docx
120 KBs Word File


