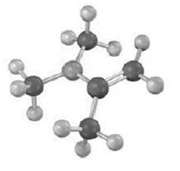
Question: The enamine prepared from acetone and dim ethylamine is shown here in its lowest-energy form. (a) What is the geometry and hybridization of the nitrogen
The enamine prepared from acetone and dim ethylamine is shown here in its lowest-energy form.
(a) What is the geometry and hybridization of the nitrogen atom?
(b) What orbital on nitrogen holds the lone pair of electrons?
(c) What is the geometric relationship between the p orbital?s of the double bond and the nitrogen orbital that holds the lone pair? Why do you think this geometry represents the minimum energy?
Step by Step Solution
★★★★★
3.49 Rating (162 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

a The nitrogen atom is sphybridized and the geometry is trigonal planar b A ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (1 attachment)
22-C-O-AN (75).docx
120 KBs Word File


