The experiment in Figure 16-8 required 5.32 mA for 964 s for complete reaction of a 5.00
Question:
The experiment in Figure 16-8 required 5.32 mA for 964 s for complete reaction of a 5.00 mL aliquot of unknown cyclohexene solution.
(a) How many moles of electrons passed through the cell?
(b) How many moles of cyclohexene reacted?
(c) What was the molarity of cyclohexene in the unknown?
Figure 16-8
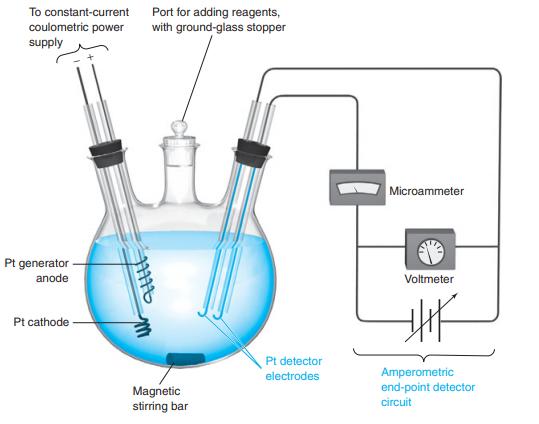
Transcribed Image Text:
To constant-current coulometric power supply Port for adding reagents, with ground-glass stopper Microammeter Pt generator anode Voltmeter Pt cathode Pt detector Amperometric end-point detector electrodes Magnetic stirring bar circuit
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
a mole b One mol e reacts with mol Br 2 w...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
The active ingredients in an antacid tablet contained only calcium carbonate and magnesium carbonate. Complete reaction of a sample of the active ingredients required 41.33 mL of 0.08750 M...
-
Explain how the amperometric end-point detector in Figure 16-8 operates. Figure 16-8 To constant-current coulometric power supply Port for adding reagents, with ground-glass stopper Microammeter Pt...
-
Does the MO energy diagram of cyclooctatetraene (Figure 16-8) appear to be a particularly stable or unstable configuration? Explain. In Figure 16.8 nonbonding line cyclobutadiene cyclooctatetraene...
-
Modify Lookup to make a program LookupAndPut that allows put operations to be specified on standard input. Use the convention that a plus sign indicates that the next two strings typed are the...
-
At the beginning of 2012, Mazzaro Company acquired equipment costing $120,000. It was estimated that this equipment would have a useful life of 6 years and a salvage value of $12,000 at that time....
-
Mountain States Electric Service is an electrical utility company serving several states in the Rocky Mountain region. It is considering replacing some of its equipment at a generating substation and...
-
If \(X_{t} \sim N(0, t)\), calculate the distribution of \(\left|X_{t} ight|\). Calculate \(\mathbf{E}\left|X_{t} ight|\) and \(V\left(\left|X_{t} ight| ight)\).
-
How would you characterize the decision-making styles of the two committees that considered the enrollment management problem? Would you characterize either of these processes as more effective or...
-
1. Define and explain the meaning of a predetermined manufacturing overhead rate that is applied in a job order costing system. ting 2. What are the advantages and disadvantages of using the cost of...
-
Procter & Gamble has been the leading soap manufacturer in the United States since 1879, when it introduced Ivory soap. However, late in 1991, its major rival, Lever Bros. (Unilever), overtook it by...
-
The sensitivity of a coulometer is governed by the delivery of its minimum current for its minimum time. Suppose that 5 mA can be delivered for 0.1 s. (a) How many moles of electrons are delivered by...
-
H 2 S(aq) can be analyzed by titration with coulometrically generated I 2 . H 2 S + I 2 + S(s) + 2H + + 2I - To 50.00 mL of sample were added 4 g of KI. Electrolysis required 812 s at 52.6 mA....
-
1. Name at least five structures that are not found in all bacteria but are important in some. 2. What characteristics are unique to gram-positive bacteria? What characteristics are unique to...
-
At what interest rate will money a. double itself in 10 years? b. triple itself in 10 years? c. quadruple itself in 10 years?
-
You have just purchased a municipal bond with a \($10\),000 par value for \($9\),500. You purchased it immediately after the previous owner received a semiannual interest payment. The bond rate is...
-
What is the effective annual interest rate for 10 percent compounded (a) semiannually, (b) every 4 months, (c) quarterly, (d) every other month, (e) monthly?
-
Shortly after completing his MS degree in engineering, Jerry Dechert wanted to initiate a retirement plan. He decided to use a 15 percent mix of blue chip individual stocks, 50 percent mutual funds,...
-
A total of \($50\),000 is borrowed and repaid with 60 monthly payments, with the first payment occurring 1 month after receipt of the \($50\),000. The stated interest rate is 6 percent compounded...
-
Is the Atlantic Ocean becoming narrower or wider? The Pacific Ocean?
-
Refrigerant-134a enters an adiabatic compressor as saturated vapor at 120 kPa at a rate of 0.3 m3/min and exits at 1-MPa pressure. If the isentropic efficiency of the compressor is 80 percent,...
-
Antibonding molecular orbitals can be used to make bonds to other atoms in a molecule. For example, metal atoms can use appropriate d orbitals to overlap with the *2p orbitals of the carbon monoxide...
-
Methyl isocyanate, CH3NCO, was made infamous in 1984 when an accidental leakage of this compound from a storage tank in Bhopal, India, resulted in the deaths of about 3,800 people and severe and...
-
(a) Methane (CH4) and the perchlorate ion are both described as tetrahedral. What does this indicate about their bond angles? (b) The NH3 molecule is trigonal pyramidal, while BF3 is trigonal planar....
-
The blue samurai, a japanese restraurant, has an asset turnover of 3.5 the total assets were 95,000 what are net sales for the blue samurai?
-
Gatekeeper Manufacturing reported 50,000 physical units that were 100% complete for direct materials during the period. In addition, the 50,000 physical units were 100% for conversion costs. In terms...
-
What red flag was overlooked on the Montague Fellowship Expense Report?

Study smarter with the SolutionInn App


