Question: The following data have been obtained for the effect of solvent composition on the solubility of a serine, an amino acid, at 10.0C: The data
The following data have been obtained for the effect of solvent composition on the solubility of a serine, an amino acid, at 10.0°C:
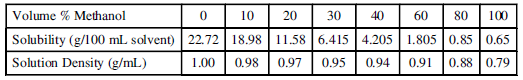
The data were obtained by mixing known volumes of methanol and water to obtain the desired solvent compositions, and then slowly adding measured amounts of serine to each mixture until no more would go into solution. The temperature was held constant at 10.0°C.
(a) Derive an expression for solvent composition expressed as mass fraction of methanol, x, as a function of volume fraction of methanol, f.
(b) Prepare a table of solubility of serine (g serine/g solution) versus mass fraction of methanol.
20 30 Solubility (g/100 mL solvent) 22.72 18.98 11.58 6.415 4.205 1.805 | 0.85 0.65 Volume % Methanol 0 10 40 80 100 Solution Density (g/mL) 0.98 1.00 0.97 0.95 0,91 0.88 | 0.79 0.94
Step by Step Solution
3.41 Rating (173 Votes )
There are 3 Steps involved in it
a Lets say mass and volume fraction of methanol are f m an... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
1359-E-C-E-C-P(1028).docx
120 KBs Word File


