Question: The following diagram shows a staged absorption column in which n-hexane (H) is absorbed from a gas into a heavy oil. A gas feed stream
The following diagram shows a staged absorption column in which n-hexane (H) is absorbed from a gas into a heavy oil. A gas feed stream containing 5.0 mole% hexane vapor and the balance nitrogen enters at the bottom of an absorption column at a basis rate of 100 mol/s. and a nonvolatile oil enters the top of the column in a ratio 2 mol oil fed/mol gas fed. The absorber consists of a series of ideal stages (see Problem 6.63), arranged so that gas flows upward and Liquid flows downward. The liquid and gas streams leaving each stage are in equilibrium with each other (by the definition of an ideal stage), with compositions related by Raoults law. The absorber operates at an approximately constant temperature T(?C) and pressure P(mm Hg). Of the hexane entering the column, 99.5% is absorbed and leaves in the liquid column effluent. At the given conditions it may be assumed that N2 is insoluble in the oil and that none of the oil vaporizes.
(a) Calculate the molar flow rates and mole fractions of hexane in the gas and liquid streams leaving the column. Then calculate the average values of the liquid and gas molar flow rates in the column, nL (mol/s) and nG (mol/s). For simplicity, in subsequent calculations use these values as the molar flow rates of the liquid and gas streams leaving each stage.
(b) Estimate the mole fraction of hexane in the gas leaving the bottom stage of the column (y1) and in the liquid entering this stage (x2).
(c) Suppose that x, and v, are the mole fractions of hexane in the liquid and gas streams leaving stage i. Derive the following formulas and verify that they yield the answers you calculated in part (b): yi = xipi (T) / P xi + 1 = xi + nG/nL(yi, ? yi-1)
(d) Create a spreadsheet to determine the number of stages (N) required to reduce the mole fraction of hexane to its required final value [calculated in part (a)] or less for P = 760torr and temperatures of 30?C, 50?C, and 70?C. The spreadsheet should have the following structure (some calculated values are shown):
Enter the values of xi. yN, nGN, nL1, and the average flow rates nG and nL calculated in parts (a) and (b). Then in the appropriate cells for the calculation at 30?C, enter the Antoine formula for the vapor pressure, the value of x1, the formula for y1 (Equation 1), and the formulas for x2 and y2 (Equations 2 and 1). Then copy the formulas into successive rows, proceeding until the value of y, is less than or equal to the calculated effluent value (y). The results (which should match the ones shown) indicate that three stages are required to achieve the specified hexane recovery at 30?C. Repeat the calculations for the other two temperatures. (You should be able to do so entirely by copying cells from one location to another on the spreadsheet.) Do not go beyond 25 stages for any temperature, whether or nor you achieve the required separation.
(e) You should have found that at 70?C and 760 mm Hg the hexane mole fraction in the vapor levels out at a value above the target value, which means that the specified separation cannot be achieved at those conditions. Explain this result. Then use your spreadsheet to determine the minimum pressure at which the target absorption can be achieved at that temperature.
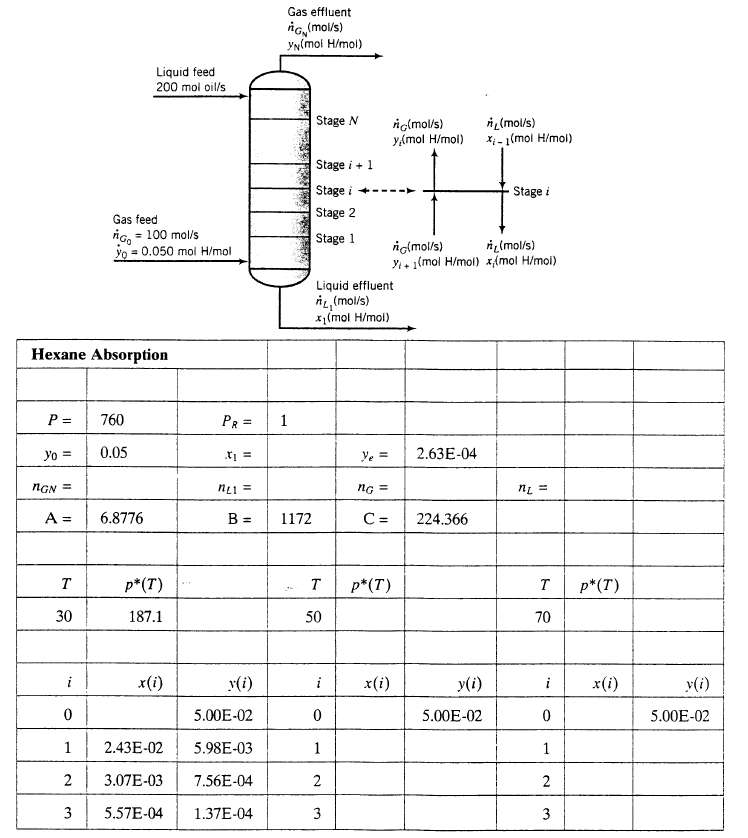
P = Hexane Absorption Yo = nGN= A = T 30 Gas feed nGo = 100 mol/s yo = 0.050 mol H/mol Liquid feed 200 mol oil/s 760 0.05 6.8776 p* (T) 187.1 x(i) PR = x = nel = y(i) 5.00E-02 5.98E-03 Gas effluent ng (mol/s) YN (mol H/mol) B = 1172 0 1 2.43E-02 2 3.07E-03 7.56E-04 3 5.57E-04 1.37E-04 1 Stage N Stage + 1 Stage Stage 2 Stage 1 T 50 i Liquid effluent , (mol/s) *(mol H/mol) 0 1 2 3 ---- Ye= ng = C = c(mol/s) y,(mol H/mol) p* (T) x(i) c(mol/s) (mol/s) Y+1(mol H/mol) x/(mol H/mol) 2.63E-04 224.366 y(i) (mol/s) x-1(mol H/mol) 5.00E-02 Stage i n = T 70 i 0 1 2 3 p* (T) x(i) y(i) 5.00E-02
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
Basis 100 mols gas feed Hhexane 200 mol oils nGN mols YN mol Hmol 1YN mol Nmol 100 mols 005 mol Hmol ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (316).pdf
180 KBs PDF File
13-E-C-E-C-P (316).docx
120 KBs Word File


