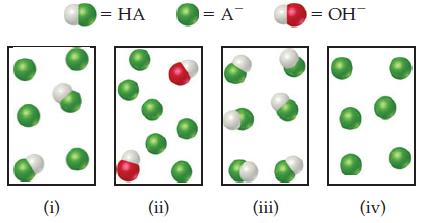
The following drawings represent solutions at various stages of the titration of a weak acid, HA, with
Question:
The following drawings represent solutions at various stages of the titration of a weak acid, HA, with NaOH. (The Na+ ions and water molecules have been omitted for clarity.) To which of the following regions of the titration curve does each drawing correspond:
(a) Before addition of NaOH,
(b) After addition of NaOH but before equivalence point,
(c) At equivalence point,
(d) After equivalence point? [Section 17.3]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:





