The isobutyl cation spontaneously rearranges to the tert-butyl cation by a hydride shift, is the rearrangement exergonic
Question:
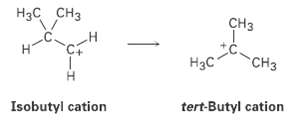
The isobutyl cation spontaneously rearranges to the tert-butyl cation by a hydride shift, is the rearrangement exergonic or endergonic? Draw what you think the transition state for the hydride shift might look like according to the Hammond postulate.
Transcribed Image Text:
Нзс снз CHз Нас "CHз Isobutyl cation tert-Butyl cation
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
The reaction is exergonic because ...View the full answer

Answered By

Manikant Kumar
I am manikant singh I have b. tech done from nit hamirpur in mechanical engineering department
After that get job in bhushan steel after douing 6 month job prepare for engineering service examination and crack two time pre and mains. My intrest in physics and teaching in coaching Institute for physics lecturer
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Draw a possible transition state for the bimolecular reaction depicted here. (The blue spheres are nitrogen atoms, and the red ones are oxygen atoms.) Use dashed lines to represent the bonds that are...
-
Draw a transition state diagram of (a) a nonenzymatic reaction and the corresponding enzyme-catalyzed reaction in which (b) S binds loosely to the enzyme and (c) S binds very tightly to the enzyme....
-
What do you think the financial-services industry will look like 20 years from now? What are the implications of your projections for its management today?
-
Figure shows three rotating, uniform disks that are coupled by belts. One belt runs around the rims of disks A and C. Another belt runs around a central hub on disk A and the rim of disk B. The belts...
-
A 25-year-old manager of a branch of a fast-food restaurant was told by her boss that she was not an effective leader. She replied, "For $29,000 a year, how can you expect me to be an effective...
-
If Dwyane Wade of the Miami Heat hits 52 out of his fi rst 100 fi eld goals in the 20132014 season, lets see how we might investigate if he is more likely than not to make a field goal? a. Based on...
-
Diet Cola and Weight Gain in Humans A study found that American senior citizens who report drinking diet soda regularly experience a greater increase in weight and waist circumference than those who...
-
A construction contractor is responsible for a project with seven key tasks. Some of the tasks can begin at any time, but others have predecessor tasks that must be completed previously. The...
-
The capital accounts of Hassan Khan and Dmitri Palovich have balances of $110,000 and $78,000, respectively, on January 1, 2014, the beginning of the fiscal year. On July 10, Khan invested an...
-
On 9 August 2017, Alfonso Garcia sold the business premises from which he has conducted his sand and gravel wholesale business for $790,000. The cost base of the premises was $520,000 when purchased...
-
Calculate the degree of unsaturation in each of the following formulas: (a) Cholesterol, C27H46O (b) DDT, C14H9C15 (c) Prostaglandin E1, C20H34O5 (d) Caffeine, C8H10N4O2 (e) Cortisone, C21H28O5 (f)...
-
Draw an energy diagram for the addition of HBr to 1-pentene. Let one curve on your diagram show the formation of 1-brornopentane product and another curve on the same diagram show the formation of...
-
What potential claims could Sarah assert against her employer? What defenses should the employer raise?
-
Delbert makes homemade salsa and sells it at his roadside produce stand. Delbert's weekly cost for making salsa can be determined using the equa- tion C=2.75j+40, where C represents the cost in dol-...
-
If courts in the United States suddenly dispensed with informal models of criminal justice and instead relied on formal procedure, what do you think would happen to the judicial system? explain and...
-
Describe various opportunity costs of attending a four-year college (assuming a full-time schedule).Given these opportunity costs, why do people choose a four-year college experience? In your own...
-
By what deed does a grantor promise that he has done nothing to encumber the property while it was in his possession, but that it does not extend to all previous owners?
-
Lachlan has a Jacaranda growing in his garden.One of the tree branches is growing towards Dan's property.Dan doesn't like this and he wants Lachlan to cut down the tree.Their respective benefits are...
-
In a robotic arm control system, the angle \(\theta(t)\) represents the robotic arm orientation, \(\omega(t)\) is the robot's angular speed, \(u(t)\) is the input force, and the output \(y(t)\) is...
-
Calculate Total Contribution Margin for the same items. Total Revenue Total Variable Costs Total Contribution Margin $50.00 a. $116.00 $329.70 b. $275.00 $14,796.00 $7,440.00 c. $40,931.25 d....
-
What is sublimation? Give a common example of sublimation.
-
Identify all of the asymmetric carbon atoms in each of the following structures. (a) (b) (c) , , H C CH CH2OH NH2 CH3
-
Give the configuration of each asymmetric atom in the following compounds. (a) (b) meso-3,4- dimethlylhexane CH H,C H OH
-
(a) Using lines, wedges, and dashed wedges as appropriate, draw perspective structures of the two stereoisomers of ibuprofen, a well-known non steroidal anti-inflammatory drug. (b) Only the S...
-
What is relational algebra? b) What are the types of relational algebra? Question No. 02 a) What is Data definition language (DDL)? b) What is Data manipulation language (DML)? Question No. 03 a)...
-
Assistant please, 1) Discuss two impacts to the Database management process by the Data definition language (DDL) and Data manipulation language (DML). 2) Share with a business partner two...
-
What does SQL stand for? What is SQL? What is SQL used for? What is PL/SQL? What is NoSQL? What is Data Definition Language (DDL)? What is Data Manipulation Language (DML)? What is Data Control...

Study smarter with the SolutionInn App


