The junction potential, Ej-, between solutions, and can be estimated with the Henderson equation: where zi is
Question:
The junction potential, Ej-, between solutions, and can be estimated with the Henderson equation:
![V Solution a | Solution B [C(B)- C;(a)] RT - In F 2 ziļu,[C(B) - C;(a)] Z,/u; C;(a) Zi E; 2 zilu, C;(B) i](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/3/4/2/7015ed9f66db65361591342699519.jpg)
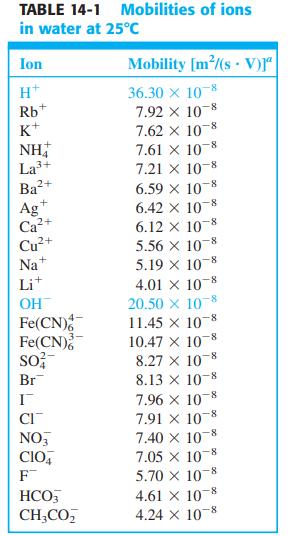
where zi is the charge of species i, ui is the mobility of species I (Table 14-1), Ci(α) is the concentration of species i in phase α , and Ci(β) is the concentration in phase . (Activity coefficients are neglected in this equation.)
(a) Using your calculator, show that the junction potential of 0.1 M HCl | 0.1 M KCl is 26.9 mV at 25°C. (Note that (RT/F) ln x = 0.059 16 log x.)
(b) Set up a spreadsheet to reproduce the result in part (a). Then use your spreadsheet to compute and plot the junction potential for 0.1 M HCl | x M KCl, where x varies from 1 mM to 4 M.
(c) Use your spreadsheet to explore the behavior of the junction potential for y M HCl | x M KCl, where y = 10-4, 10-3, 10-2, and 10-1 M and x = 1 mM or 4 M.
Step by Step Answer:






