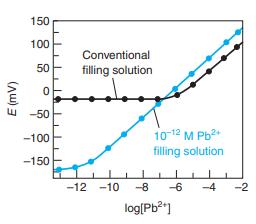
Question: The Pb 2+ ion buffer used inside the electrode for the colored curve in Figure 14-27 was prepared by mixing 1.0 mL of 0.10 M
The Pb2+ ion buffer used inside the electrode for the colored curve in Figure 14-27 was prepared by mixing 1.0 mL of 0.10 M Pb(NO3)2 with 100.0 mL of 0.050 M Na2EDTA. At the measured pH of 4.34,α γ4- = 1.46 × 10-8 (Equation 11-4). Show that [Pb2+] = 1.4 × 10-12 M.
Figure 14-27
Equation 11-4
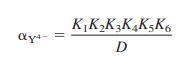
150 100 Conventional 50 filling solution -50 10-12 M Pb2 filling solution -100 -150 -12 -10 -8 -6 -4 -2 log[Pb?"] (Aw) 3
Step by Step Solution
3.48 Rating (158 Votes )
There are 3 Steps involved in it
There is a large excess of EDTA in the buffer We expect essentially ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2070).docx
120 KBs Word File


