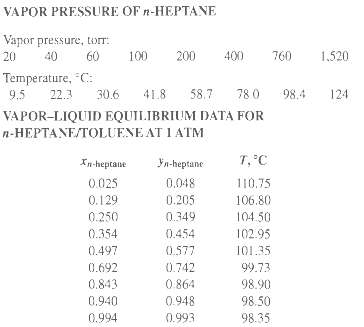
The vapor pressure of toluene is given in Exercise 4.8, and that of n-heptane is given in
Question:
The vapor pressure of toluene is given in Exercise 4.8, and that of n-heptane is given in the accompanying table.
(a) Plot an x-y equilibrium diagram for this system at 1 atm by using Raoult's and Dalton's laws.
(b) Plot the T-x bubble-point curve at 1 atm.
(c) Plot a and K-values versus temperature.
(d) Repeat part (a) using the arithmetic average value of a, calculated from the two extreme values.
(e) Compare your x-y and T-x-y diagrams with the following experimental data of Steinhauser and White.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





