The wavelengths of maximum absorption and emission of anthracene in Figure 17-22 are approximately 357 and 402
Question:
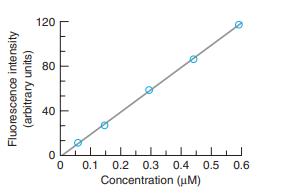
The wavelengths of maximum absorption and emission of anthracene in Figure 17-22 are approximately 357 and 402 nm. Molar absorptivities at these wavelengths are εex 9.0 × 103M-1 cm-1 and εem = 5 × 101 M-1 cm-1. Consider a fluorescence experiment in Figure 17-21 with cell dimensions b1 = 0.30 cm, b2 = 0.40 cm, and b3 = 0.5 cm. Calculate the relative fluorescence intensity with Equation 17-12 as a function of concentration over the range 10-8 to 10-3 M. Explain the shape of the curve. Up to approximately what concentration is fluorescence proportional to concentration (within 5%)? Is the calibration range in Figure 17-23 sensible?
Figure 17-21
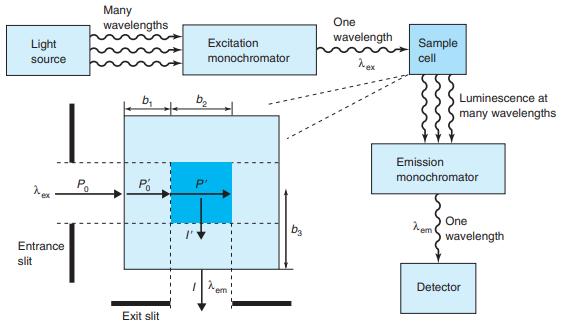
Figure 17-22
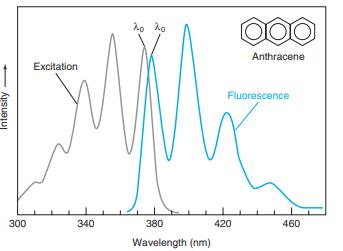
Figure 17-23
Equation 17-12
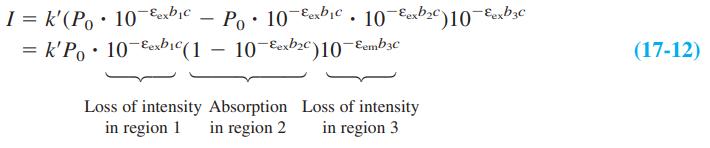
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





