Question: Ti 3+ is to be generated in 0.10 M HClO 4 solution for coulometric reduction of azobenzene. At the counter electrode, water is oxidized, and
Ti3+ is to be generated in 0.10 M HClO4 solution for coulometric reduction of azobenzene.
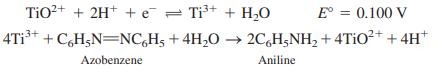
At the counter electrode, water is oxidized, and O2 is liberated at a pressure of 0.20 bar. Both electrodes are made of smooth Pt, and each has a total surface area of 1.00 cm2. The rate of reduction of the azobenzene is 25.9 nmol/s, and the resistance of the solution between the generator electrodes is 52.4 Ω.
(a) Calculate the current density (A/m2) at the electrode surface. Use Table 16-1 to estimate the overpotential for O2 liberation.
(b)Calculate the cathode potential (versus S.H.E.) assuming that [TiO2+] surface [TiO2+]bulk 0.050 M and [Ti3+]surface 0.10 M.
(c) Calculate the anode potential (versus S.H.E.).
(d) What should the applied voltage be?
TiO?+ + 2H+ + e = Ti+ + H,0 E = 0.100 V 4T1* + C,H;N=NC,H; + 4H,0 2C,H;NH, + 4TIO* + 4H* Azobenzene Aniline
Step by Step Solution
3.39 Rating (165 Votes )
There are 3 Steps involved in it
a 4 259 b c C6H5NNC6H5 4H 4e 2C6H5NH2 Electron flow electrons d current de... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2117).docx
120 KBs Word File


