Two mechanisms for the transport of gas components through a porous membrane that are not discussed in
Question:
Two mechanisms for the transport of gas components through a porous membrane that are not discussed in Section 14.3 or illustrated in Figure are (1) partial condensation in the pores by some components of the gas mixture to the exclusion of other components and subsequent transport of the condensed molecules through the pore, and (2) selective adsorption on pore surfaces of certain components of the gas mixture and subsequent surface diffusion across the pores. In particular, Rao and Sircar [48] have found that the latter mechanism provides a potentially attractive means for separating hydrocarbons from hydrogen for low-pressure gas streams. In porous-carbon membranes with continuous pores 4-15 A in diameter, little pore void space is available for the Knudsen diffusion of hydrogen when the hydrocarbons are selectively adsorbed. Typically, the membranes are not more than 5 pm in thickness. Measurements at 295.1 K of permeabilities for five pure components and a mixture of the five components are as follows:
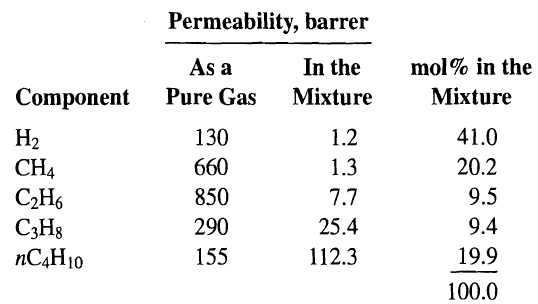
A refinery waste gas mixture of the preceding composition is to be processed through such a porous-carbon membrane. If the pressure of the gas is 1.2 atm and an inert sweep gas is used on the permeate side such that partial pressures of feed gas components on that side are close to zero, determine the permeate composition on a sweep-gas-free basis when the composition on the upstream-pressure side of the membrane is that of the feed gas. Explain why the component permeabilities differ so drastically between experiments with the pure gas and the gas mixture.
Step by Step Answer:






