Question: Using the curved-arrow notation, derive a resonance structure for the allyl anion (shown here) which shows that the two carbon-carbon bonds an identical bond order
Using the curved-arrow notation, derive a resonance structure for the allyl anion (shown
here) which shows that the two carbon-carbon bonds an identical bond order of 1.5 and that the unshared electron pair (and negative charge) is shared equally by the two terminal carbons.
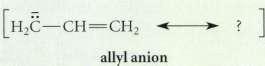
H2C CH CH2 allyl anion
Step by Step Solution
3.22 Rating (169 Votes )
There are 3 Steps involved in it
H CHCH ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
902-C-O-O-S (120).docx
120 KBs Word File


