Question: refer book chapter 6 if necessary Chapter 6: Van der Waals Forces | Intermolecular and Surface Forces, 3rd Edition by Jacob N. Israelachvili (oreilly.com) Intermolecular
refer book chapter 6 if necessary Chapter 6: Van der Waals Forces | Intermolecular and Surface Forces, 3rd Edition by Jacob N. Israelachvili (oreilly.com)
Intermolecular and Surface Forces | ScienceDirect
6.4 (only in the case where r1=r2=r3=?)
The simple treatment used to derive the London equation for the dispersion interaction energy between two isolated molecules A and B (Section 6.1) can be extended to include the effect of a third molecule C. This arises because an additional component of the electric field from A reaches B by "reflection" from C (Figure 6.5). Consider three identical molecules of polarizabilities a and ionization potentials J = hv sitting at the corners of a triangle with sides of length r, ro, and rs. Ignoring numerical factors, derive a simple approximate expression in terms of , I, r), fo, and rs for the additional three-body interaction energy. On the basis of this equation, obtain a rough estimate for the magnitude of the three-body energy relative to the two-body (pair) interaction for the case when the three molecules are in contact?that is, when r| = r2 = r3 = a. Explain, in qualitative terms, why the three-body contribution is negative (favorable) when three identical molecules are in line, as in Figure 6.5, but positive when in the symmetrical triangular configuration. [For further reading on three-, four-, and many-body effects, see Margenau and Kestner (1971).]
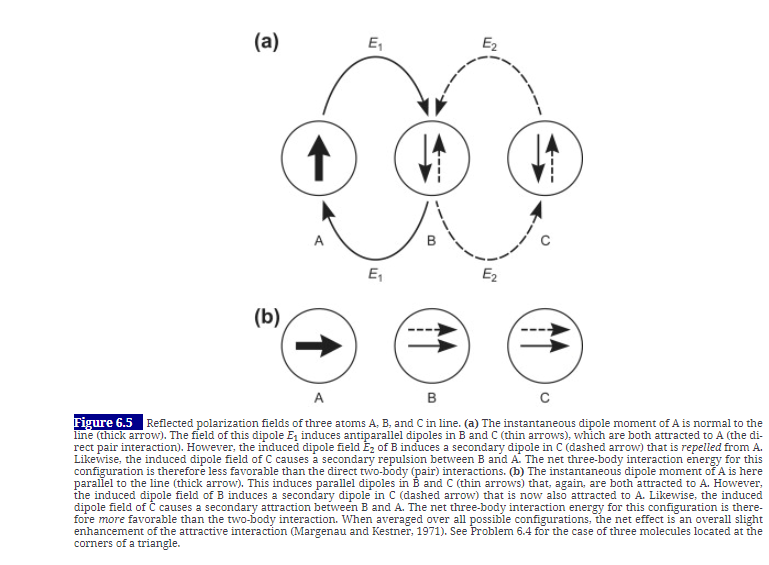
(a) E1 A B C E1 (b) A B C Figure 6.5 Reflected polarization fields of three atoms A, B, and Cin line. (a) The instantaneous dipole moment of A is normal to the line (thick arrow). The field of this dipole E, induces antiparallel dipoles in B and C (thin arrows), which are both attracted to A (the di- rect pair interaction). However, the induced dipole field E of B induces a secondary dipole in C (dashed arrow) that is repelled from A. Likewise, the induced dipole field of C causes a secondary repulsion between B and A. The net three-body interaction energy for this configuration is therefore less favorable than the direct two-body (pair) interactions. (b) The instantaneous dipole moment of A is here parallel to the line (thick arrow). This induces parallel dipoles in B and C (thin arrows) that, again, are both attracted to A. However, the induced dipole field of B induces a secondary dipole in C (dashed arrow) that is now also attracted to A. Likewise, the induced dipole field of C causes a secondary attraction between B and A. The net three-body interaction energy for this configuration is there- fore more favorable than the two-body interaction. When averaged over all possible configurations, the net effect is an overall slight enhancement of the attractive interaction (Margenau and Kestner, 1971). See Problem 6.4 for the case of three molecules located at the corners of a triangle
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


