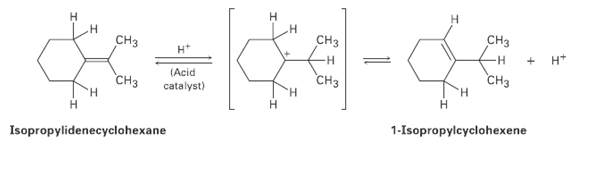
When isopropylidenecyclohexane is treated with strong acid at room temperature, isomerization occurs by the mechanism shown below
Question:
When isopropylidenecyclohexane is treated with strong acid at room temperature, isomerization occurs by the mechanism shown below to yield 1-iso- propylcyclohexenc: At equilibrium, the product mixture contains about 30% isopropylidenecyclohexane and about 70% 1-isopropylcyclohexene.
(a) What is an approximate value of for the reaction?
(b) Since the reaction occurs slowly at room temperature, what is its approximate ΔG++?
(c) Draw an energy diagram for the reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





