Question: Which of the cations in Table 12.3 would you predict to form iodides having the cesium chloride crystal structure? Justify yourchoices. Ionic Radius Ionic Radius
Which of the cations in Table 12.3 would you predict to form iodides having the cesium chloride crystal structure? Justify yourchoices.
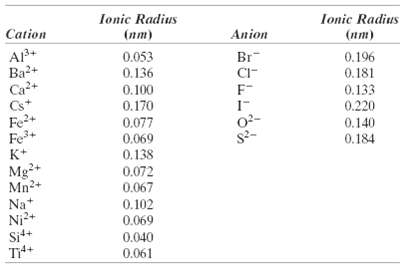
Ionic Radius Ionic Radius Cation (nm) Anion () 0.053 0.136 Br 0.196 Ba2+ Ca+ Cs+ Fe2+ Fe+ K+ CI- 0.181 0.100 0.133 0.220 0.170 0.077 0.069 0.138 02- 0.140 0.184 Mg+ Mn2+ 0.072 0.067 Na+ Ni2+ Sit+ 0.102 0.069 0.040 0.061
Step by Step Solution
3.45 Rating (177 Votes )
There are 3 Steps involved in it
We are asked to cite the cations in Table 123 which would form iodi... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (464).docx
120 KBs Word File


