Question: Without looking at Table 24.2, rank the following compounds in order of ascending basicity. (a) p-Nitro aniline, p-aminobenzaldehyde, p-Bromoaniline (b) p-Chloroanilinc, p-aminoacetophenone, p-methyl aniline (c)
Without looking at Table 24.2, rank the following compounds in order of ascending basicity.
(a) p-Nitro aniline, p-aminobenzaldehyde, p-Bromoaniline
(b) p-Chloroanilinc, p-aminoacetophenone, p-methyl aniline
(c) p-(Trifluoromethyl) aniline, p-methyl aniline, p-(fluoro methyl)aniline
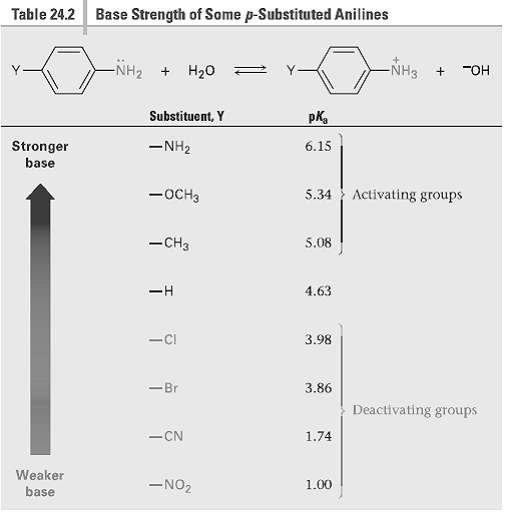
Table 24.2 Base Strength of Some p-Substituted Anilines -NH2 + H20 NH3 "OH Substituent, Y pKa -NH2 Stronger base 6.15 5.34 Activating groups -OCH3 -CH3 5.08 4.63 3.98 -Ci -Br 3.86 Deactivating groups -CN 1.74 Weaker -NO2 1.00 base
Step by Step Solution
3.41 Rating (170 Votes )
There are 3 Steps involved in it
The basicity order of substituted arylamines is the same as their react... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-A (55).docx
120 KBs Word File


