A 5,000-kg/h aqueous solution of 20 wt% Na2SO4 is fed to an evaporative crystallizer operating at 60?C.
Question:
A 5,000-kg/h aqueous solution of 20 wt% Na2SO4 is fed to an evaporative crystallizer operating at 60?C. Equilibrium data are given in Figure. If 80% of the Na2SO4 is to be crystallized, calculate:
(a) The kilograms of water that must be evaporated per hour
(b) The crystallizer pressure intom
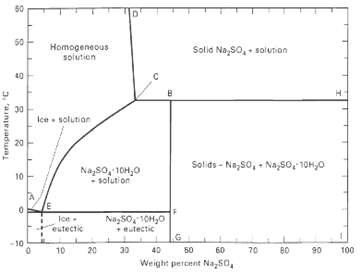
Transcribed Image Text:
50 50 Homogeneous solution Solid Na,so, soluton 40 30 Ice solunior 20 Solids - Na,50, - Na,So, 10H,0 Nazso, 10H,0 solution 10 Ice eutectic Nay50, 10H,0 + eutectic -10 40 60 10 20 50 70 80 06 100 Weight percent Na,50, 3. ainjeadua
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 37% (8 reviews)
a From Fig at 60 o C the stable crystals are Na 2 SO 4 with no water of crystallization The solubili...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Experimental liquid-phase activity-coefficient data are given in Exercise 2.23 for the ethanol/benzene system at 45oC. Estimate and plot diffusion coefficients for both ethanol and benzene over the...
-
Demand and supply of laptop computers are given in Figure 6P-4. The quantity of laptops is given in thousands. Suppose the government provides a $300 subsidy for every laptop computer that consumers...
-
Unit step response data are given in Table E7.15 for a process with gain K = 2. Fit the data to a first order model with no time delay. Next use linear regression to fit a first-order discrete-time...
-
How can we define tectonic stress?
-
Bergio Bar & Grill has had $5,300 of sales today. Of these, $2,700 were paid by 62 customers with their debit cards, $900 was paid by customers with cash, and the rest was paid with credit cards. The...
-
What information should be disclosed about property, plant, and equipment in the consolidated financial statements?
-
Use these data to answer Questions 2a and 2b. a. Using either a calculator or Excel, compute the Pearson correlation coefficient. b. Interpret the relationship between these variables using the...
-
Sailor Products, Inc., manufactures flotation vests in San Diego, California. Sailor Products' contribution margin income statement for the most recent month contains the following data: Suppose...
-
An ambulance siren that is at rest emits a sound wave of a wavelength of 1m. What is the wavelength that an observer at rest, with respect to the street, measures when the ambulance siren is moving...
-
The FBI posted a flyer on all American Airlines. The flyer indicated, "The Federal Government will pay $500,000 to any person providing information leading to the arrest of any airline customer,...
-
Repeat Example 4.17 for 90% evaporation of the water.
-
Calculate the dew-point pressure, secondary dew-point pressure, and bubble-point pressure of the following mixtures at 50C, assuming that the liquid aromatics and water are mutually insoluble: (a) 50...
-
Simplify. (+11) - (+14)
-
A chromatin-remodeling complex may a. change the locations of nucleosomes. b. evict histones from DNA. c. replace standard histones with histone variants. d. do all of the above.
-
The process of RNA interference may lead to a. the degradation of an mRNA. b. the inhibition of translation of an mRNA. c. the synthesis of an mRNA. d. both a and b.
-
How can methylation affect transcription? a. It may prevent the binding of regulatory transcription factors. b. It may enhance the binding of regulatory transcription factors. c. It may promote the...
-
Which of the following function(s) is/are carried out by piRITS or piRISC? a. Inhibits transcription of TEs b. Causes the degradation of TE RNA c. Causes chromosome breakage d. Both a and b are...
-
Which of the following is a function of SRP? a. Pausing translation of a polypeptide via an ER signal sequence b. Binding to an SRP receptor in the ER membrane c. Docking the ribosome over a channel...
-
Which of the following is a possible explanation for pleiotropy? a. The expression of a single gene can affect cell function in more than one way. b. A gene may be expressed in different cell types...
-
Write the given system without the use of matrices. D) - ()- d (x sin t + 8 (2+ 1)
-
What are the main benefits and challenges of a diverse workforce? In what ways do both organizations and workers benefit from this diversity, if at all?
-
A charge of 100 lbmol of 35 mol% n-hexane, 35 mol% nheptane, and 30 mol% n-octane is to be distilled at 1 atm in a batch rectifier, consisting of a still-pot, a column, and a total condenser, at a...
-
A charge of 200 kmol of a mixture of 40 mol% A, 50 mol% B, and 10 mol% C, with aA;C = 2:0 and aB;C = 1:5, is to be separated in a batch rectifier with three theoretical stages, including the still...
-
A charge of 100 kmol of an equimolar mixture of A, B, and C, with aA;B = 2 and aA;C = 4, is distilled in a batch rectifier containing four theoretical stages, including the still-pot. If holdup can...
-
Seinfeld: The Apartment JERRY: Boys, boys. HAROLD: Oh, Jerry. JERRY: I slid the rent under your door, Harold. Did you get it? HAROLD: Yeah, yeah. Hey, Jerry, would you like anything from Mrs....
-
Let f be twice differentiable with f(0) = 6, f(1) = 8, and f'(1) = 7. Evaluate the following integral. [ = 0 0 xf" (x)dx
-
Bearwood Inc. completed Job No.D50 during 2021. The job cost sheet listed the following: Job No. D50 Costs for 12,000 units produced: Direct materials Direct labor Manufacturing overhead applied...

Study smarter with the SolutionInn App


