Question: A cumulene is a compound with three adjacent double bonds. Draw an orbital picture of a cumulene. What kind of hybridization do the two central
A cumulene is a compound with three adjacent double bonds. Draw an orbital picture of a cumulene. What kind of hybridization do the two central carbon atoms have? What is the geometric relationship of the sub-stituents on one end to the sub-stituents on the other end? What kind of isomerism is possible? Make a model to help see theanswer.
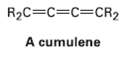
R2C=C=C=CR2 A cumulene
Step by Step Solution
3.47 Rating (170 Votes )
There are 3 Steps involved in it
sp2 R R spsp o bond a bonds sp sp o bond bonds sp spsp o bond sp R3 R4 This simple... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-O-S (46).docx
120 KBs Word File


