A hydrocarbon vapor-liquid mixture at 250?F and 500 psia contains N2, H2S, CO2, and all the normal
Question:
A hydrocarbon vapor-liquid mixture at 250?F and 500 psia contains N2, H2S, CO2, and all the normal paraffins from methane to heptane. Use Figure to estimate the K-value of each component in the mixture. Which components will have a tendency to be present to a greater extent in the equilibriumvapor?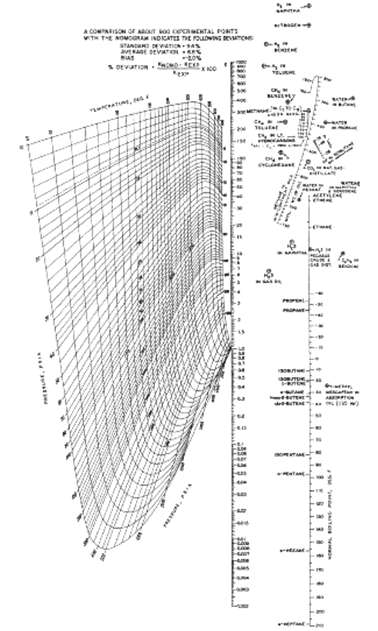
Transcribed Image Text:
A COAISON G t o cecaENTAL PONTE VT TE NOMCO INDICATES TE FouwINE Evmc STANNI DEVAnoSAS 13D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
If the Kvalue is 10 tendency is for vapor phase Using Fig ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
(a) What are the mole fractions of each component in a mixture of 15.08 g of O2, 8.17 g of N2, and 2.64 g of H2? (b) What is the partial pressure in atm of each component of this mixture if it is...
-
A 250 L rigid tank contains methane at 500 K, 1500 kPa. It is now cooled down to 300 K. Find the mass of methane and the heat transfer using a) ideal gas and b) the methane tables.
-
A 250 L rigid tank contains methane at 500 K, 1500 kPa. It is now cooled down to 300 K. Find the mass of methane and the heat transfer using a) ideal gas and b) the methane tables.
-
Consider Problem 13.28. The solvent MDEA becomes rich in acid gases. To recycle this solvent, it is first heated to 90C in exchanger E-2001 and then sent to the top stage of the stripper T-2002 as...
-
On June 30, $150,000 of five-year, 10% Plaza bonds are issued at $138,960 to yield a market interest rate of 12%. Interest is payable semi-annually each June 30 and December 31. (a) Record the...
-
Rewrite the BNF of Example 3.4 to add the ++ and -- unary operators of Java. Data from Example 3.4: A | B|C + | * | ( ) |
-
Explain how consent differs among competent patients, minors, guardians, and incompetent patients.
-
A water wave traveling in a straight line on a lake is described by the equation Where y is the displacement perpendicular to the undisturbed surface of the lake? (a) How much time does it take for...
-
1. One of your users was recently issued a new Windows 10 computer that was configured by a new administrator in your department. The user reports she received an access denied message while trying...
-
A piece of string is connected between two adjacent houses to form a clothesline. The houses are separated by d = 22 m, and both ends of the string are the same height off the ground. A sweater is...
-
Isopropanol, containing 13 wt% water, can be dehydrated to obtain almost pure isopropanol at a 90% recovery by azeotropic distillation with benzene. When condensed, the overhead vapor from the column...
-
Acetone, a valuable solvent, can be recovered from air by absorption in water or by adsorption on activated carbon. If absorption is used, the conditions for the streams entering and leaving are as...
-
The following data represent the length of time (in minutes) between eruptions of Old Faithful in Yellowstone National Park. Time (minutes) .... Frequency 40-49 ........ 8 50-59 ........ 44 60-69...
-
What types of benefits are generally not compensated for in workers compensation systems?
-
Which of the following acts constitute the tort of bad faith? a. The insurance carrier unreasonably delays payment. b. The insurance carrier acts unconscionably toward its insured. c. The insurance...
-
An action in which the insured is seeking payment from their insurer is called a a. class action suit. b. first-party claim. c. bad faith claim. d. breach of contract action.
-
What is a reservation of rights?
-
When and for what purpose would an insurer file a declaratory judgment action against its insured?
-
The volume-cost-profit relationships provide management with a simplified framework for organising its thinking on a number of problems. Discuss.
-
After Theorem 1.5 we note that multiplying a row by 0 is not allowed because that could change a solution set. Give an example of a system with solution set S0 where after multiplying a row by 0 the...
-
An experimental station wishes to test whether a growth hormone will increase the yield of wheat above the average value of 100 units per plot produced under currently standard conditions. Twelve...
-
What is a reasonable value for the optimal absorption factor when designing an absorber? Does that same value apply to the optimal stripping factor when designing a stripper?
-
What is the difference between an operating line and an equilibrium curve?
-
For a given recovery of a key component in an absorber or stripper, does a minimum absorbent or stripping agent flow rate exist for a tower or column with an infinite number of equilibrium stages?
-
When are harassing behaviors unwelcome? What is the liability to organizations for harassment?
-
Franklin on vacation for several weeks. While Franklin was away his neighbour, Samantha, decided to weed and maintain his large vegetable and flower gardens, as well as cut the grass in his large...
-
Why is the fact that a hospital operates an emergency room open to all persons regardless of ability to pay is a significant factor the IRS considers when determining if the hospital provides a...

Study smarter with the SolutionInn App


