Consider Problem 13.28. The solvent MDEA becomes rich in acid gases. To recycle this solvent, it is
Question:
Consider Problem 13.28. The solvent MDEA becomes rich in acid gases. To recycle this solvent, it is first heated to 90°C in exchanger E-2001 and then sent to the top stage of the stripper T-2002 as shown in Figure P13.29. Develop a nonequilibrium-stage model of the stripper with 20 theoretical stages, a partial-vapor condenser, and a kettletype reboiler. Reflux ratio (mole basis) = 0.7 and bottoms/feed ratio (mole basis) = 0.97. The pressure in the condenser is 1.4 atm, and the pressure drop through the entire column should be calculated. Consider the ionic reactions in all the stages, including the condenser and reboiler. It can be assumed that all ionic reactions reach equilibrium in the condenser and reboiler because of the longer residence times. Assume that the tray hardware is similar to the absorber. The mass transfer and the ionic reactions in the liquid and vapor films can be modeled similarly to Problem 13.28. For this problem determine the following: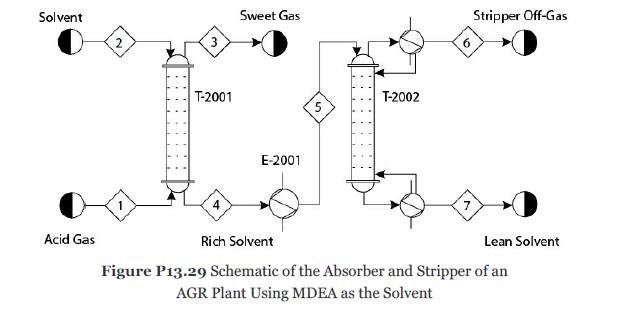
1. What are the duties of the stripper and reboiler?
2. What are the compositions of Streams 6 and 7?
3. What operating condition(s) of the stripper would you change if you wanted to increase the purity of Stream 7?
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





