A light-hydrocarbon feed stream contains 45.4 kmol/h of propane, 136.1 kmol/h of isobutane, 226.8 kmol/h of n-butane,
Question:
A light-hydrocarbon feed stream contains 45.4 kmol/h of propane, 136.1 kmol/h of isobutane, 226.8 kmol/h of n-butane, 181.4 kmol/h of isopentane, and 317.4 kmol/h of n-pentane. This stream is to be separated in a sequence of three distillation columns, similar to that in Figure, into four products: (1) propane-rich, (2) isobutane-rich, (3) n-butane-rich, and (4) combined pentanesrich. However, the distillate from the first column is to be the propane-rich product; the distillate from Column 2 is to be the isobutane-rich product; and the distillate from Column 3 is to be the n-butane-rich product, with the combined pentanes being the bottoms from Column 3. The recovery of each main component in each product is to be 98%. For example, 98% of the propane in the feed stream is to appear in the propane-rich product, and 98% of the combined pentanes in the feed stream is to appear in the bottoms product from Column 3.(a) Draw a process-flow diagram, similar to Figure.(b) Complete a material balance for each column and summarize the results in a table similar to Table 1.5. To complete the material balance, you will have to make some assumptions about the flow rates of: (1) isobutane in the distillates for Columns 1 and 3 and (2) n-butane in the distillates for Columns 1 and 2, consistent with the specified recoveries. Assume that propane will not be found in the distillate from Column 3 and pentanes will not be found in thedislillate from Column 2.(c) Calculate the mol% purities of each of the products and summarize your results in a table similar to Table 1.7, but without the specifications, which are not givenhere.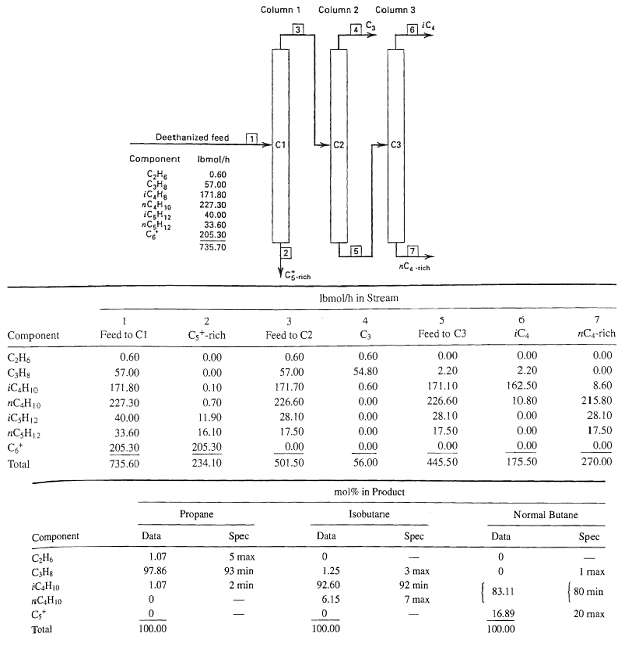
Step by Step Answer:






