A natural gas containing 95 mole% methane and the balance ethane is burned with 20.0% excess air.
Question:
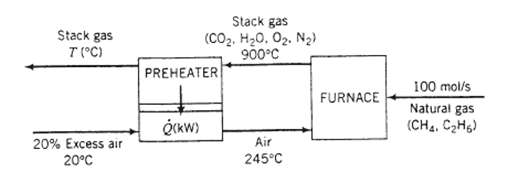
A natural gas containing 95 mole% methane and the balance ethane is burned with 20.0% excess air. The stack gas which contains no unburned hydrocarbons or carbon monoxide leaves the furnace at 900?C and 1.2 atm and passes through a heat exchanger. The air on its way to the furnace also passes through the heat exchanger, entering it at 20?C and leaving it at 245?C.
(a) Taking as a basis 100 molls of the natural gas fed to the furnace calculate the required molar flow rate of air, the molar flow rate and composition of the stack gas, the required rate of heat transfer in the pre-heater, Q (write an energy balance on the air), and the temperature at which the stack gas leaves the pre-heater (write an energy balance on the stack gas). Note: The problem statement does not give you the fuel feed temperature. Make a reasonable assumption and state why your final results should be nearly independent of what you assume.
(b) What would Q be if the actual feed rate of the natural gas were 350 SCMH [standard cubic meters per hour, m3 (STP)/h]? Scale up the flowchart of part (a) rather than repeating the entire calculation.?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





