Question: A salt A is soluble in a solvent S. A conductivity meter used to measure the solute concentration in A?S solutions is calibrated by dissolving
A salt A is soluble in a solvent S. A conductivity meter used to measure the solute concentration in A?S solutions is calibrated by dissolving a known quantity of A in S. adding more S to bring the solution volume to a fixed value, and noting the conductivity meter reading. The data given below are taken at 30?C:
The following experiment is performed. One hundred sixty grams of A is dissolved in S at 30?C S is added until a final solution volume of 500 mL is obtained. The solution is cooled slowly to 0?C while being stirred and is maintained at this temperature long enough for crystallization to be complete. The concentration of A in the supernatant liquid is then measured with the conductivity meter, yielding R = 17.5. The solution is next reheated in small temperature increments. The last crystal is observed to dissolve at 10.2?C. A specific gravity of 1.10 may be assumed for all A?S solutions.
(a) Derive an expression for C (g A/mL solution) in terms of R.
(b) Calculate the solubility?s (g A/100 g S) at 10.2?C and 0?C and the mass of solid crystals in the beaker at 0?C.
(c) If half the solvent in the flask were to evaporate at 0?C, how much more A would come out of solution?
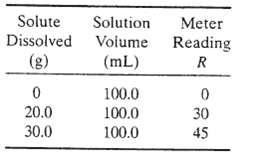
Solute Dissolved (g) 0 20.0 30.0 Solution Volume (mL) 100.0 100.0 100.0 Meter Reading R 0 30 45
Step by Step Solution
3.51 Rating (158 Votes )
There are 3 Steps involved in it
a g A dissolved R 0 30 CA mL solution CA Plot CA vs R CA R1... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (328).docx
120 KBs Word File


