Question: A solution contains two volatile liquids A and B. Complete the following table, in which the symbol indicates attractive intermolecular forces. Deviation from Attractive
A solution contains two volatile liquids A and B. Complete the following table, in which the symbol → indicates attractive intermolecular forces.
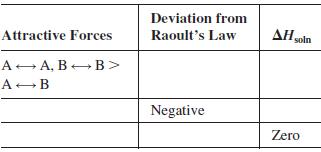
Deviation from Attractive Forces Raoult's Law AH soln A A, B B> A-B Negative Zero
Step by Step Solution
3.35 Rating (173 Votes )
There are 3 Steps involved in it
The completed table is shown below The first row represents a Case 1 situat... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
932-C-O-C (2386).docx
120 KBs Word File


