A spherical stainless steel vessel at 93?C contains 45 kg of water initially at the same temperature.
Question:
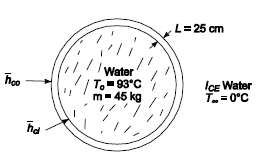
A spherical stainless steel vessel at 93?C contains 45 kg of water initially at the same temperature. If the entire system is suddenly immersed in ice water, determine (a) the time required for the water in the vessel to cool to 16?C, and (b) the temperature of the walls of the vessel at that time. Assume that the heat transfer coefficient at the inner surface is 17 W/(m2 K), the heat transfer coefficient at the outer surface is 22.7 W/(m2 K), and the wall of the vessel is 2.5 cm thick.GIVENA spherical stainless steel vessel of water is suddenly immersed in ice waterInitial temperature of vessel and water (Ti) = 93?CMass of water in the vessel (m) = 45 kgThe inner heat transfer coefficient hci = 17 W/(m2 K)The outer heat transfer coefficient hco = 22.7 W/(m2 K)The vessel wall thickness (L) = 2.5 cm = 0.025 mASSUMPTIONSThe water in the vessel is well mixed, therefore its temperature is uniformThe vessel is completely filled withwater
Step by Step Answer:

Principles of heat transfer
ISBN: 978-0495667704
7th Edition
Authors: Frank Kreith, Raj M. Manglik, Mark S. Bohn





