Question: According to Table 17.3, the oxide coating that forms on silver should be nonprotective, and yet Ag does not oxidize appreciably at room temperature and
According to Table 17.3, the oxide coating that forms on silver should be nonprotective, and yet Ag does not oxidize appreciably at room temperature and in air. How do you explain this apparentdiscrepancy?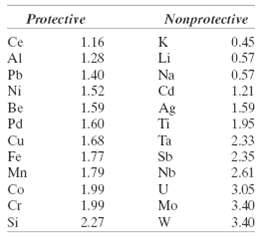
Nonprotective Protective Ce 1.16 0.45 Al Li 0.57 1.28 Na Cd 0.57 1.21 Pb 1.40 1.52 Ni Be Pd 1.59 1.60 1.59 1.95 Ag Ti Cu Ta 2.33 2.35 2.61 1,68 1.77 1.79 Fe Sb Mn Nb Co 1.99 3.05 3.40 Cr 1.99 Mo Si 2.27 3.40
Step by Step Solution
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Silver does not oxidize appreciably at room temperat... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (671).docx
120 KBs Word File


