Question: Although aldehydes and ketones are weak acids, their a-hydrogens are rnore than 30 pKa units more acidic than the hydrogen's of alkanes. Using polar effects
Although aldehydes and ketones are weak acids, their a-hydrogens are rnore than 30 pKa units more acidic than the hydrogen's of alkanes.
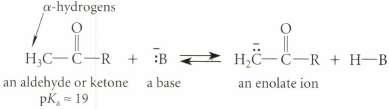
Using polar effects and resonance effects in your argument, explain the enhanced acidity of aldehydes and ketones.
-hydrogens an aldehyde or ketone pK 19 a base an enolate ion
Step by Step Solution
★★★★★
3.44 Rating (179 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The conjugatebase enolate ion is stabilized by the polar electro... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (1 attachment)
902-C-O-O-S (505).docx
120 KBs Word File


