Question: Although anti periplanar geometry is preferred for E2 reactions, it isn?t absolutely necessary. The deuterated bromo compound shown here reacts with strong base to yield
Although anti periplanar geometry is preferred for E2 reactions, it isn?t absolutely necessary. The deuterated bromo compound shown here reacts with strong base to yield an un-deuterated alkene. Clearly, a syn elimination has occurred. Make a molecular model of the reactant, and explain the result.
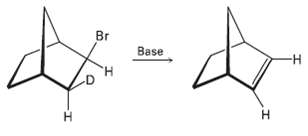
Br Base -
Step by Step Solution
3.29 Rating (178 Votes )
There are 3 Steps involved in it
One of the steric requirements of E2 elimination is the need ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-E-R (154).docx
120 KBs Word File


