At 25C and 101 kPa, 2 mol of a gas containing 35 mol% propylene in propane is
Question:
At 25°C and 101 kPa, 2 mol of a gas containing 35 mol% propylene in propane is equilibrated with 0.1 kg of silica gel adsorbent. Using the equilibrium data of Figure, calculate the moles and composition of the gas adsorbed and the equilibrium composition of the gas not adsorbed.
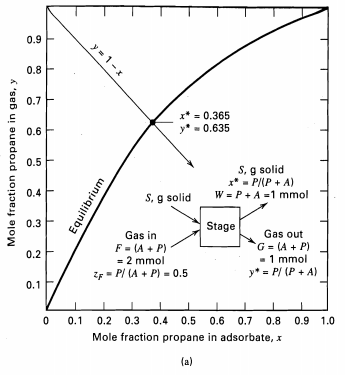
Transcribed Image Text:
0.9 y = 1-x 0.8 0.7 x* = 0.365 - 0.635 0.6 0.5 S, g solid x* - P/(P + A) W = P+A =1 mmol 0.4 S, g solid 0.3 Stage Gas out G = (A + P) - 1 mmol y* = P/ (P + A) Gas in F = (A + P) = 2 mmol 0.2 ZF- P/ (A + P) = 0.5 0.1 0.1 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 Mole fraction propane in adsorbate, x (a) Mole fraction propane in gas, y wnuqinb3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
Let y and x be the mole fractions of P in the vapor and ad...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
A gas containing 50 mol% propylene in propane is to be separated with silica gel having the equilibrium properties shown in Figure. The final products are to be 90 mol% propylene and 75 mol% propane....
-
A 30.0-liter cylinder of a gas containing 97.0 mole% CO and 3.0% CO2 is delivered to your plant. You sign the receipt for it, noting that the gauge on the tank reads 2000 psi. Several days later you...
-
The temperature of 3.00 mol of a gas with Cv = 6.00cal/mol K is to be raised 50.0 K. If the process is at constant volume, what are? (a) The energy transferred as heat Q, (b) The work W done by the...
-
Why do joints tend to be evenly spaced rather than clustered?
-
The following information is for Hudson Corporation. HUDSON CORPORATION Bank Reconciliation August 31 ..........................................................................$41,720 Add: Deposits...
-
Graph the system of linear inequalities and indicate the solution set. x + y < 4 3x + 2y 6
-
An option based on a variable that is not traded is called a real option or sometimes a soft option. Find the projection price of the soft option with the following parameters and compare with the...
-
A cylindrical disk of wood Figure 14.34 Exercise 14.23 weighing 45.0 N and having a diameter of 30.0 cm floats on a cylinder of oil of density 0.850 g/cm3 (Fig. 14.34). The cylinder of oil is 75.0 cm...
-
what is the most efficient way to see the sources and targets related to a transaction?
-
Greenville has provided the following information from its General Fund Revenues and Appropriations/ Expenditure/Encumbrances subsidiary ledgers for the fiscal year ended. Assume the beginning fund...
-
Nitrogen at 760 torr and 300?C contains 10 mol% anthraquinone (A). If this gas is cooled to 200?C, calculate the percent desublimation of A. Vapor pressure data for solid A are as follows: These data...
-
Repeat Example 4.17 for 90% evaporation of the water.
-
Calculate cp, cv, e, and h for (a) The stagnation point conditions given in Prob. 7.1 (b) Air at standard sea level conditions (If you do not remember what standard sea level conditions are, find...
-
Rather than enhancing the Sorted List ADTs by adding a member function IsThere, you decide to write a client function to do the same task. 1. Write the specifications for this function. 2. Write the...
-
Write a member function Copy of the Stack ADT, assuming that the stack named in the parameter list is copied into self.
-
Indicate whether a stack would be a suitable data structure for each of the following applications. 1. A program to evaluate arithmetic expressions according to the specific order of operators 2. A...
-
Define and implement a counted stack, which inherits from StackType.
-
Implement the class constructor, destructor, and copy constructor for the circular linked list class.
-
When would it be appropriate to recognize a gain or loss on the abandonment or retirement of a well or individual item of equipment? a. If the well or individual item of equipment constitutes a part...
-
In Problems, solve each system of equations. x + 2y + 3z = 5 y + 11z = 21 5y + 9z = 13
-
Explain the effects of the global economic crisis on employment.
-
What is the basic equation for computing the rate of mass transfer through a membrane? Explain each of the four factors in the equation and how they can be exploited to obtain high rates of mass...
-
For the commercial application of membrane separators discussed at the beginning of this chapter, calculate the permeabilities of hydrogen and methane in barrer units.
-
What kinds of materials are membranes made from? Can a membrane be porous or nonporous? What forms pores in polymer membranes?
-
Individual Retirement Account (IRA) Bonds Mutual fund Stocks Futures Defined contribution plans What is it? Level of Risk and Potential Return Minimum investment? Easy to start or stop?
-
1. A company purchased machinery in 2015 for $400,000. Its value in 2018 was $320,000. Assuming the resale value decreases exponentially, what will the value be in 2020? As a part of your solution,...
-
ROA of a company is 8.57%, Total assets end of the year of 2021 are $9.6 million, ROE is 14% and Profit margin of 19.9% what is the firms value of net income? and what is stockholders equity?

Study smarter with the SolutionInn App


