Question: At what position and on what ring do you expect nitration of 4-bromo- biphenyl to occur? Explain, using resonance structures of the potentialintermediates. 4-Bromobiphenyl Br
At what position and on what ring do you expect nitration of 4-bromo- biphenyl to occur? Explain, using resonance structures of the potentialintermediates.
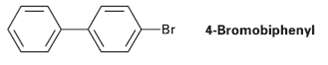
4-Bromobiphenyl Br
Step by Step Solution
3.48 Rating (164 Votes )
There are 3 Steps involved in it
Attack occurs on the unsubstituted ring because bromine ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-B (44).docx
120 KBs Word File


