Question: (a) Find the initial composition of a COCO2 gas mixture necessary to establish an equilibrium oxygen partial pressure of pO2=1103 atm at 1473K. The standard
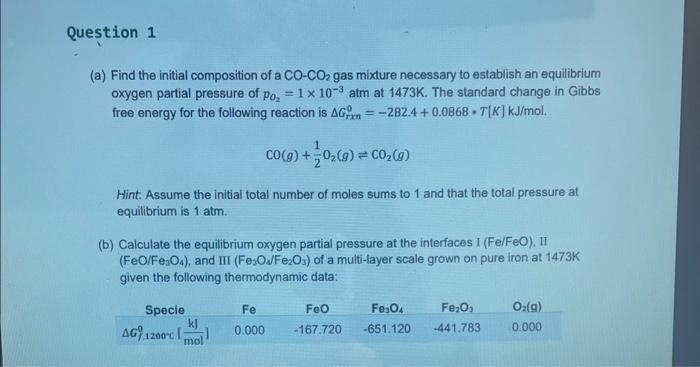
(a) Find the initial composition of a COCO2 gas mixture necessary to establish an equilibrium oxygen partial pressure of pO2=1103 atm at 1473K. The standard change in Gibbs free energy for the following reaction is Grxn0=282.4+0.0868T[K]kJ/mol. CO(g)+21O2(g)CO2(g) Hint: Assume the initial total number of moles sums to 1 and that the total pressure at equilibrium is 1atm. (b) Calculate the equilibrium oxygen partial pressure at the interfaces I (Fe/FeO), II ( FeO/Fe3O4), and III (Fe3O2/Fe2O3) of a multi-layer scale grown on pure iron at 1473K given the following thermodynamic data: (a) Find the initial composition of a COCO2 gas mixture necessary to establish an equilibrium oxygen partial pressure of pO2=1103 atm at 1473K. The standard change in Gibbs free energy for the following reaction is Grxn0=282.4+0.0868T[K]kJ/mol. CO(g)+21O2(g)CO2(g) Hint: Assume the initial total number of moles sums to 1 and that the total pressure at equilibrium is 1atm. (b) Calculate the equilibrium oxygen partial pressure at the interfaces I (Fe/FeO), II ( FeO/Fe3O4), and III (Fe3O2/Fe2O3) of a multi-layer scale grown on pure iron at 1473K given the following thermodynamic data
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


