Chlorate (CO - 3 ), chlorite (CO - 2 ) , bromate (BrO - 3 ), and
Question:
Chlorate (CO-3), chlorite (CO-2) , bromate (BrO-3), and iodate (IO-3) can be measured in drinking water at the 1-ppb level with 1% precision by selected reaction monitoring. Chlorate and chlorite arise from ClO2 used as a disinfectant. Bromate and iodate can be formed from Br-or I- when water is disinfected with ozone (O3). For the highly selective measurement of chlorate, the negative ion selected by Q1 in Figure 21-26 is m/z 83 and the negative ion selected by Q3 is m/z 67. Explain how this measurement works and how it distinguishes CIO-3 from CIO-2, BrO-3 , and IO-3
Figure 21-6
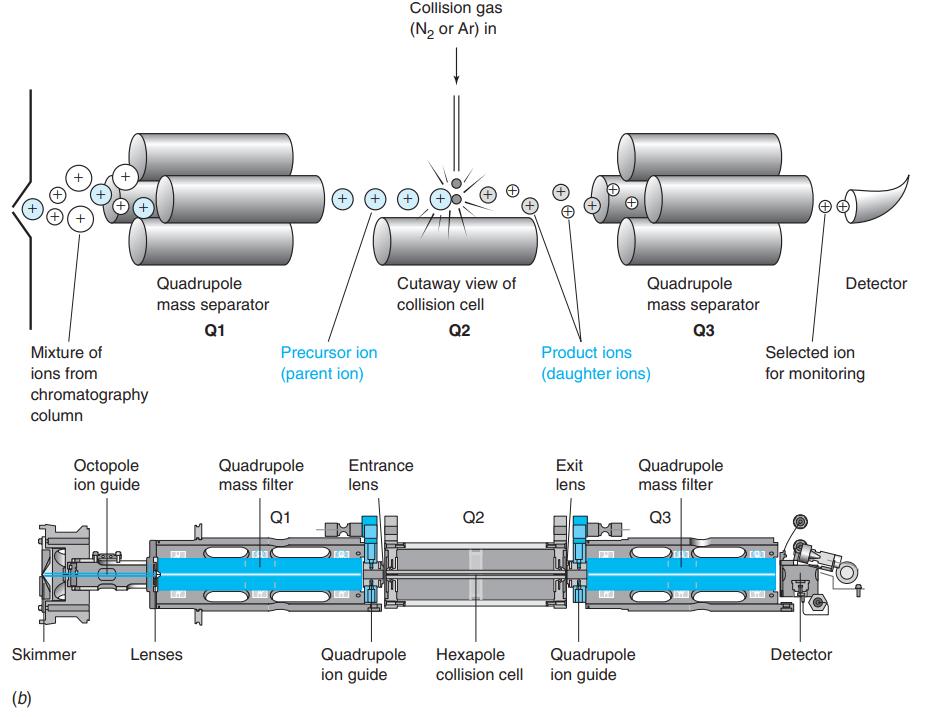
Collision gas (N2 or Ar) in + Quadrupole mass separator Cutaway view of Quadrupole mass separator Detector collision cell Q1 Q2 аз Mixture of Precursor ion Product ions Selected ion ions from (parent ion) (daughter ions) for monitoring chromatography column Octopole ion guide Quadrupole Entrance Exit Quadrupole mass filter mass filter lens lens Q1 Q2 Q3 Skimmer Lenses Quadrupole ion guide Нехарole Quadrupole ion guide Detector collision cell (b)
Step by Step Answer:
Selected reaction monitoring chooses the molecular ion ...View the full answer



Related Video
For this experiment take the bottle halves. Place the bottle upside down in another half of the bottle. Take a piece of cotton and put the cotton in the bottle first. Then pour sand into it. After adding sand pour fine activated charcoal. You can see the making of Homemade ACTIVATED CHARCOAL in the previous video. I’ll give you the link to the video in the description. After making a 1/2 Inch layer of charcoal put 1 inch of gravel layer and then put 2 inches of pebble layer. Repeat the layer-making process in the same manner once again. After this pour dirty water on the top of the bottle. The water we get at the end is free of impurities.
Students also viewed these Chemical Engineering questions
-
The maximum allowable concentration of lead in drinking water is 9.0 ppb.
-
Verify that, in SI units, B/t can be measured in volts-in other words, that 1 Wb/s = 1 V.
-
The true weight of an object can be measured in a vacuum, where buoyant forces are absent. An object of volume V is weighed in air on a balance with the use of weights of density P. If the density of...
-
What sort of debates or experiences would get overlooked if professionals and researchers ignored these distinctions? How would you classify the following? An assigned expatriate who falls in love...
-
You are provided with the following information for Perkins Inc. for the month ended October 31, 2014. Perkins uses a periodic method for inventory. Instructions (a) Calculate (i) ending inventory ,...
-
What type of exploratory research design (observation, projective technique, in-depth interview, focus group, case study, ethnography, netnography, ZMET) would you suggest for each of the following...
-
A PI (Polarization Index) Test of a motor winding is performed and the 10-minute ratio is 3.0. The curve is a perfect parabolic shape. What is the verdict?
-
(Term Modification with GainDebtors Entries) Use the same information as in E14-21 above except that American Bank reduced the principal to $1,900,000 rather than $2,400,000. On January 1, 2014,...
-
The driver of a car traveling at 31.9 m/s applies the brakes and undergoes a constant deceleration of 1.12 m/s 2 . How many revolutions does each tire make before the car comes to a stop, assuming...
-
Erica and Bob participate in a friendly Hackathon that allows each to solve one question a day out of the three offered. There will be one easy, one medium and one hard question, with points awarded...
-
Phytoplankton at the ocean surface maintain the fluidity of their cell membranes by altering their lipid (fat) composition when the temperature changes. When the ocean temperature is high, plankton...
-
In isotope dilution, a known amount of an unusual isotope (called the spike) is added to an unknown as an internal standard for quantitative analysis. After the mixture has been homogenized, some of...
-
The total factory overhead for Diva-nation is budgeted for the year at $180,000, divided into four activities: cutting, $18,000; sewing, $36,000; setup, $96,000; and inspection, $30,000. Diva-nation...
-
What does the term Best practice often refer to? A standardized set of deliverables, like plans, reports, and checklists. A set of Tools and techniques that a project manager should master. The...
-
Response on how you feel about this opinion, agree or disagree with supporting points The transactional leadership style focuses on process and control from a leader and is characterized by a strict...
-
Regardless of whether consumers are aware or not, many products in the United States are developed with products of genetic engineering. For example, 90% of soybeans and 88% of animal feed corn are...
-
This writer feels that you have made some great points in this discussion post! One point that stuck out to this writer is the importance of seeking professional help when a person is grieving and...
-
As we have discussed in class in-office, remote, and hybrid presence models are currently being evaluated by knowledge workers post COVID. Samsung company's outline a presence model justifying your...
-
The American Time Use Survey is a survey of adult Americans conducted by the Bureau of Labor Statistics. The purpose of the survey is to learn how Americans allocate their time in a day. As a...
-
An access route is being constructed across a field (Figure Q8). Apart from a relatively firm strip of ground alongside the field's longer side AB, the ground is generally marshy. The route can...
-
(a) What are the advantages and disadvantages of using a narrower open tubular column? (b) What are the advantages and disadvantages of using a longer open tubular column? (c) What are the advantages...
-
(a) What types of solutes are typically separated with a poly(dimethylsiloxane)-coated open tubular column? (b) What types of solutes are typically separated with a poly(ethylene glycol)-coated open...
-
(a) What are the advantages and disadvantages of temperature programming in gas chromatography? (b) What is the advantage of pressure programming?
-
do not use chatgpt or any other ai tool. A monopolist with a linear demand curve will have a marginal revenue curve with intercept and slope as the demand curve..
-
7. Consider the figure below. HO -C pka-COOH-2.19 pka-NH2 = 9.67 pka-sidechain 4.25 CHCH2C OH a. What amino acid is this? (1) b. Is it in the R or S configuration? (2) c. Draw the three forms of the...
-
2. Draw the structure of the missing major organic product(s) or reactant(s) in each of the transformations below including showing stereochemistry when appropriate. For reactions that produce an...

Study smarter with the SolutionInn App


